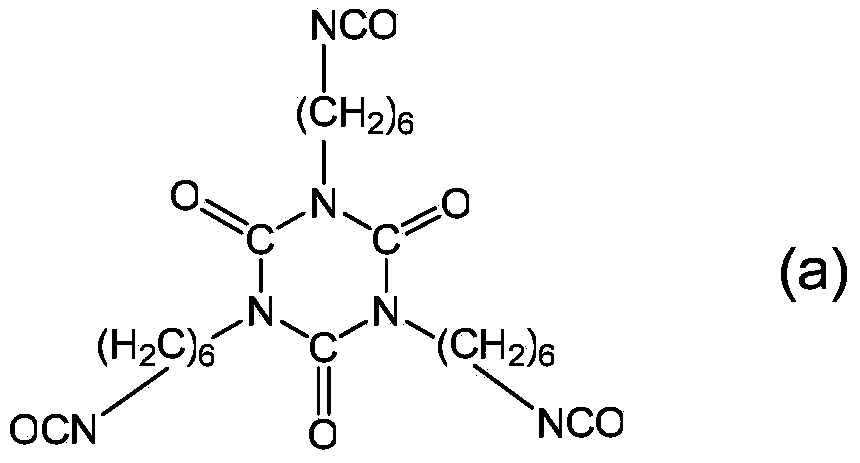
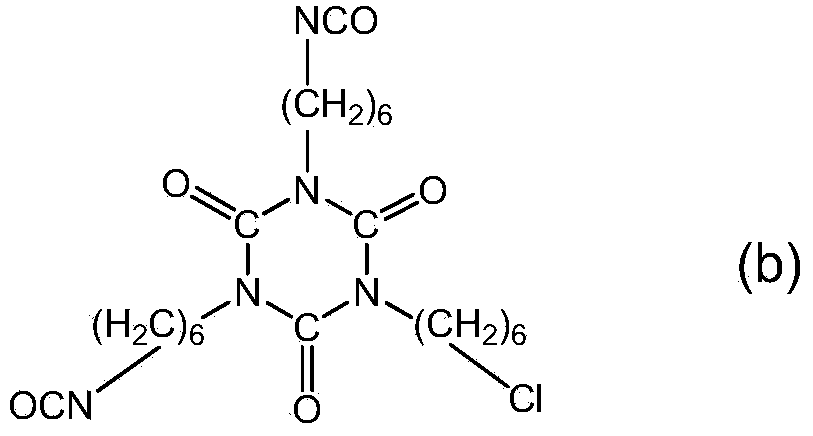
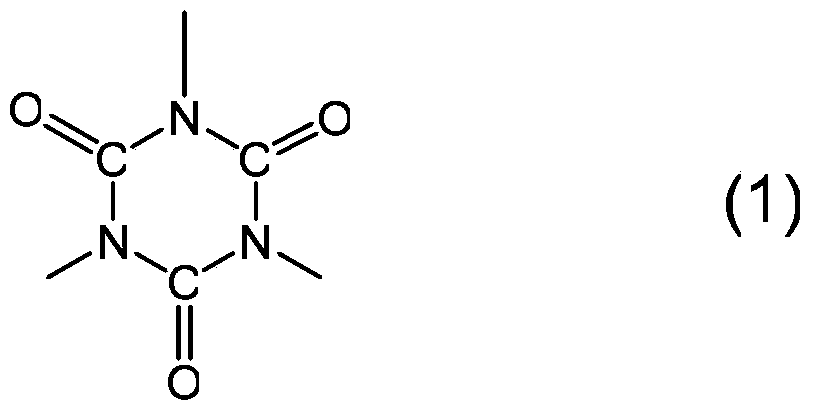
Polyisocyanate composition
A polyisocyanate and isocyanurate technology, applied in the direction of polyurea/polyurethane coatings, coatings, etc., can solve the problems of poor curing and achieve the effect of inhibiting coloration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0147] Hereinafter, the present invention will be described in more detail based on examples and comparative examples, but the present invention is not limited to the following examples.
[0148]
[0149] The viscosity was measured at 23° C. using an E-type viscometer (manufactured by Tokyo Keiki Co., Ltd.). In the measurement, a standard rotor (1°34'×R24) was used. The rotation speed is as follows.
[0150] 100rpm (less than 128mPa·s)
[0151] 50rpm (over 128mPa·s and less than 256mPa·s)
[0152] 20rpm (over 256mPa·s and less than 640mPa·s)
[0153] 10rpm (over 640mPa·s and less than 1280mPa·s)
[0154] 5rpm (over 1280mPa·s and less than 2560mPa·s)
[0155] In addition, the non-volatile content of the polyisocyanate composition produced in each Example and each comparative example mentioned later was investigated by the method described below, and when the value was 98 mass % or more, it measured it as it was. When it is less than 98% by mass, the concentration of the...
Synthetic example 1
[0250] (Synthesis Example 1: Synthesis of Catalyst)
[0251] Into a 500 mL eggplant-shaped flask replaced with nitrogen, 200 g (0.116 mol) of tetramethylammonium hydroxide (10% by mass methanol solution) (manufactured by Tokyo Chemical Industry Co., Ltd.) was charged at room temperature, and capric acid was added dropwise with a dropping funnel (manufactured by Tokyo Chemical Industry Co., Ltd.) 12.1 g (tetramethylammonium hydroxide / capric acid=1 / 1.1 (molar ratio)), and stirred at room temperature for 30 minutes. Thereafter, methanol was distilled off under conditions of 10 Torr, 50° C., and 30 minutes. 32 g of n-butanol was added thereto to obtain a 50% by mass butanol solution of tetramethylammonium caprate. Then, 40 g of n-butanol was further added to 10 g of the 50 mass % butanol solution of tetramethylammonium caprate, to obtain a 10 mass % butanol solution of tetramethylammonium caprate. Further, 15 g of n-butanol was further added to 10 g of this 10 mass % butanol sol...
Synthetic example 2
[0252] (Synthesis Example 2: Synthesis of Acrylic Polyol)
[0253] Into a four-necked flask equipped with a stirrer, a thermometer, and a condenser, 120.0 g of "SOLVESSO #150" (an aromatic solvent manufactured by Exxon Corporation Chemical Co., Ltd.) and 60.0 g of xylene were charged, and after the inside was replaced with nitrogen, the temperature was raised to 120°C. Thereafter, (meth)acrylic monomers (128.8 g of methyl methacrylate, 84.8 g of n-butyl acrylate, 80.0 g of cyclohexyl methacrylate, 74.4 g of 2-hydroxyethyl methacrylate) were added dropwise over 2 hours. g, styrene 32.0g) and benzoyl peroxide 8.0g, make it carry out stirring reaction. After completion of the dropwise addition, the reaction was further continued at 120° C. for 4 hours to obtain acrylic polyol Ac-1.
[0254] The obtained acrylic polyol Ac-1 had a non-volatile content of 70% by mass, a hydroxyl value of 80 mgKOH / g (value calculated from the feed ratio to the resin component, JIS K1557), and a gla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com