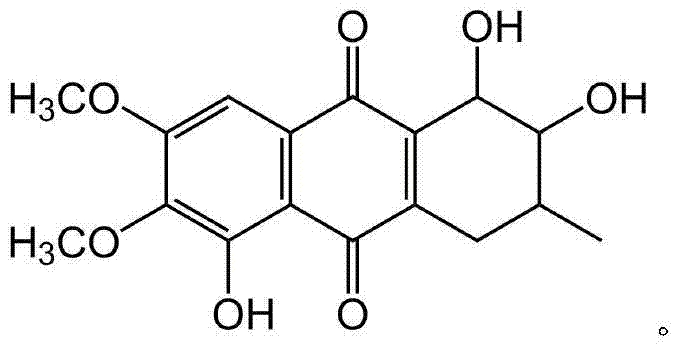
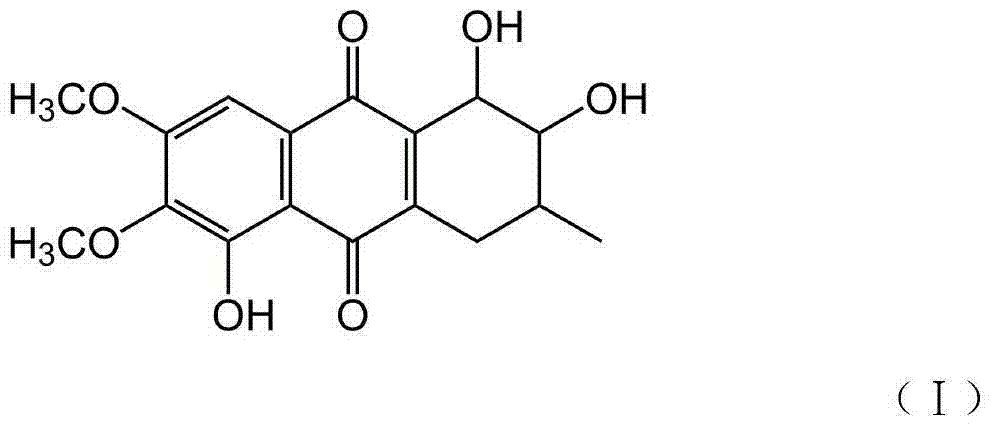
Naphthaquinone compound derived from marine microbes, and preparation method and application thereof
A compound, naphthoquinone technology, applied in the field of naphthoquinone compounds, can solve the problems of large toxic and side effects, limited dose increase and clinical treatment effect, etc., achieves low application cost, broad development and application prospects, improved targeting and high efficiency sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the preparation of naphthoquinone compound CWL-168
[0026] Preparation of bacterial classification: use seawater PDA culture medium, high-temperature sterilization, make plate, place under normal temperature state, inoculate Penicillium chrysogenum (can be purchased from China Marine Microorganism Culture Collection and Management Center, preservation number: MCCC 3A00005) mycelia, cultured statically for 4-5 days at 28°C, as a strain; the seawater PDA medium consists of 200g of potatoes, 20g of glucose, 15-20g of agar, and 1L of seawater;
[0027] Preparation of fermented seed liquid: put seawater PDA liquid culture medium into multiple Erlenmeyer flasks respectively, after high temperature sterilization, inoculate the above strains, shake and cultivate at 180rpm / min at 28°C for 2-3 days, and use the culture as seed liquid;
[0028] Inoculation: adopt solid fermentation method, prepare fermentation bottle, add rice solid fermentation medium, sterilize a...
Embodiment 2
[0034] Embodiment 2: MTT method assays the antitumor activity of naphthoquinone compound CWL-168
[0035] In this example, an in vitro cytotoxicity test was used to determine the antitumor activity of the compound CWL-168 by detecting the survival rate of human or mouse cancer cells cultured in vitro after adding CWL-168.
[0036] The selected cell lines are: human non-small cell lung cancer H1299, A549, human large cell lung cancer H460, human small cell lung cancer H446, mouse melanoma B16, B16-F10, human breast cancer MD-MBA-231, MCF7, human gastric cancer MGC803 , human pancreatic cancer PANC-1, human cervical cancer HeLa, human osteosarcoma U2OS, human liver cancer HepG2, human colon cancer HCT116, and human laryngeal cancer HEP2. MTT method was used to determine the half-inhibitory concentration IC when the survival rate (or death rate) of each tumor cell was 50%. 50 (The above cells can all be purchased from ATCC).
[0037] The half-inhibitory concentration IC50 of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com