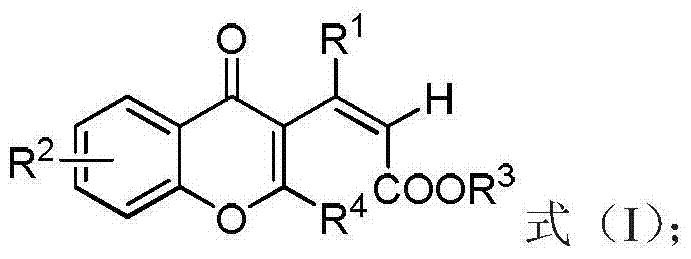
Chromone derivative and synthesis method thereof
A synthetic method and derivative technology, applied in organic chemistry and other fields, can solve the problems of limited substrates and cumbersome synthesis of raw materials, and achieve the effects of simple post-processing, good yield, and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Synthesis of 2-methyl-3-(cis-1-phenyl-2-formyl ethyl) enyl chromone
[0029]
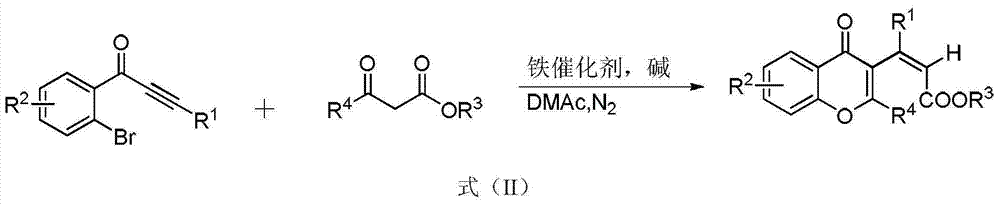
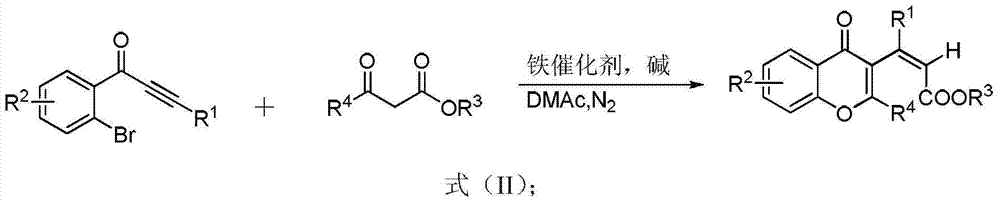
[0030] 1-(2'-bromophenyl)-2-phenylethynyl ketone, ethyl acetoacetate, Cs 2 CO 3 , Fe(ClO 4 ) 3 9H 2 O, N, N-dimethylacetamide, the consumption of raw material is o-bromoacetylenone 1-(2'-bromophenyl)-2-phenylethynyl ketone 0.3mmol, ethyl acetoacetate 0.3mmol, inorganic base ( Cs 2 CO 3 )0.6mmol, solvent 3ml, iron salt (Fe(ClO 4 ) 3 9H 2 O) 0.06mmol, reacted at 100°C for 2 hours to obtain the target product formula (IA), a yellow liquid, and the separation yield was 81%.
[0031] NMR data: 1 H NMR (400MHz, CDCl 3 , Me 4 Si) δ1.02-1.09(m, 3H), 2.13(s, 3H), 3.97-4.03(m, 2H), 6.55(s, 1H), 7.18-7.30(m, 4H), 7.32-7.36(m , 1H), 7.39-7.41(m, 2H), 7.52-7.57(m, 1H), 8.13(d, J=7.2Hz, 1H); 13 C NMR (100.6MHz, CDCl 3 , Me 4 Si): δ13.64, 18.68, 59.95, 117.81, 120.73, 121.28, 123.06, 124.97, 126.35, 126.99, 128.93, 129.88, 133.58, 138.47, 148.55, 156.30, 162.69, 1675.23
[003...
Embodiment 2
[0033] Example 2: Synthesis of 2-methyl-3-(cis-1-4'-chloro-phenyl-2-formyl ethyl) enyl chromone
[0034]
[0035]1-(2'-bromophenyl)-2-(4'-chloro)phenylethynyl ketone, ethyl acetoacetate are selected for o-bromoacetylenone, 1,3-dicarbonyl compound, inorganic base, iron salt and solvent respectively 、Cs 2 CO 3 , Fe(ClO 4 ) 3 9H 2 O, N, N-dimethylacetamide, the amount of raw materials is o-bromoacetylenone 1-(2'-bromophenyl)-2-(4'-chloro)phenylethynyl ketone 0.3mmol, ethyl acetoacetate 0.3mmol, 0.6mmol of inorganic base, 3ml of solvent, and 0.06mmol of iron salt were reacted at 100°C for 3 hours to obtain the target product formula (IB), a yellow liquid, and the separation yield was 72%.
[0036] NMR data: 1 H NMR (400MHz, CDCl 3 , Me 4 Si): δ1.14-1.19(m, 3H), 2.23(s, 3H), 4.05-4.14(m, 2H), 6.61(s, 1H), 7.27-7.32(m, 2H), 7.34-7.39( m, 1H), 7.42-7.46(m, 3H), 7.63-7.67(m, 1H), 8.20(d, J=7.2Hz, 1H); 13 C NMR (100.6MHz, CDCl 3 , Me 4 Si) δ13.57, 18.60, 59.99, 117.78, 1...
Embodiment 3
[0038] Example 3: Synthesis of 2-methyl-3-(cis-1-4'-methyl-phenyl-2-formyl ethyl) enyl chromone
[0039]
[0040] 1-(2'-bromophenyl)-2-(4'-methyl)phenylethynyl ketone, ethyl acetoacetate Esters, Cs 2 CO 3 , Fe(ClO 4 ) 3 9H 2 O, N, N-dimethylacetamide, the amount of raw materials is o-bromoacetylenone 1-(2'-bromophenyl)-2-(4'-methyl)phenylethynyl ketone 0.3mmol, ethyl acetoacetate 0.3mmol of ester, 0.6mmol of inorganic base, 3ml of solvent, and 0.06mmol of iron salt were reacted at 100°C for 3 hours to obtain the target product formula (IC), a yellow liquid, and the separation yield was 75%.
[0041] NMR data: 1 H NMR (400MHz, CDCl 3 , Me 4 Si): δ1.13-1.18(m, 3H), 2.22(s, 3H), 2.32(s, 3H), 4.02-4.14(m, 2H), 6.62(s, 1H), 7.11-7.15(m, 2H), 7.27-7.44(m, 4H), 7.59-7.64(m, 1H), 8.21(d, J=7.6Hz, 1H); 13 C NMR (100.6MHz, CDCl 3 , Me 4 Si): δ13.57, 18.53, 20.76, 59.74, 117.70, 119.50, 121.23, 122.99, 124.81, 126.22, 126.82, 129.58, 133.44, 135.43, 140.11, 148.44, 156.21,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com