Operation rinsing water solution as well as preparation method and application thereof
A technology for flushing aqueous solutions and aqueous solutions, which can be used in pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., and can solve problems such as poor stability and toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
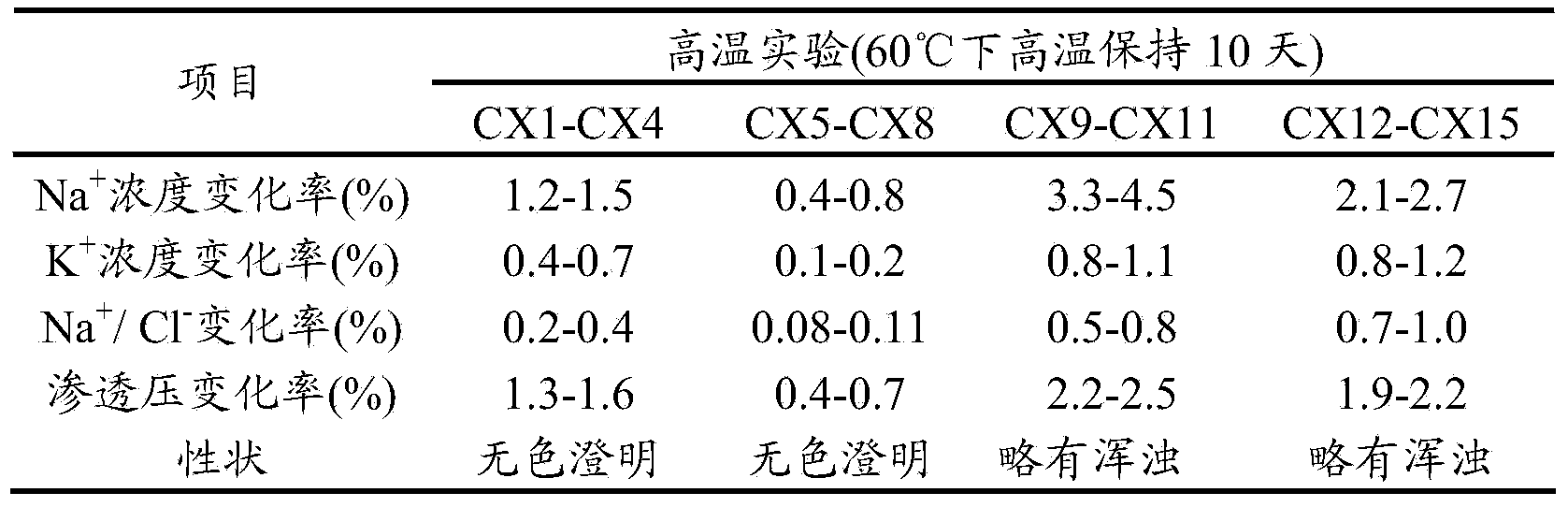
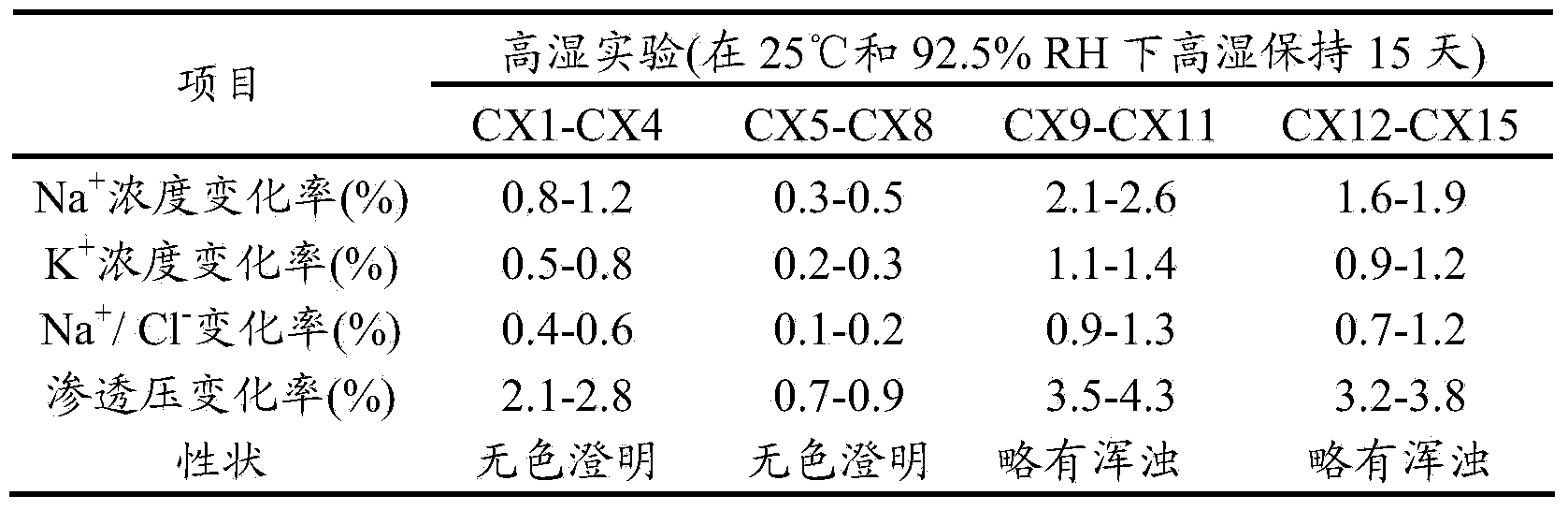
Examples
Embodiment 1
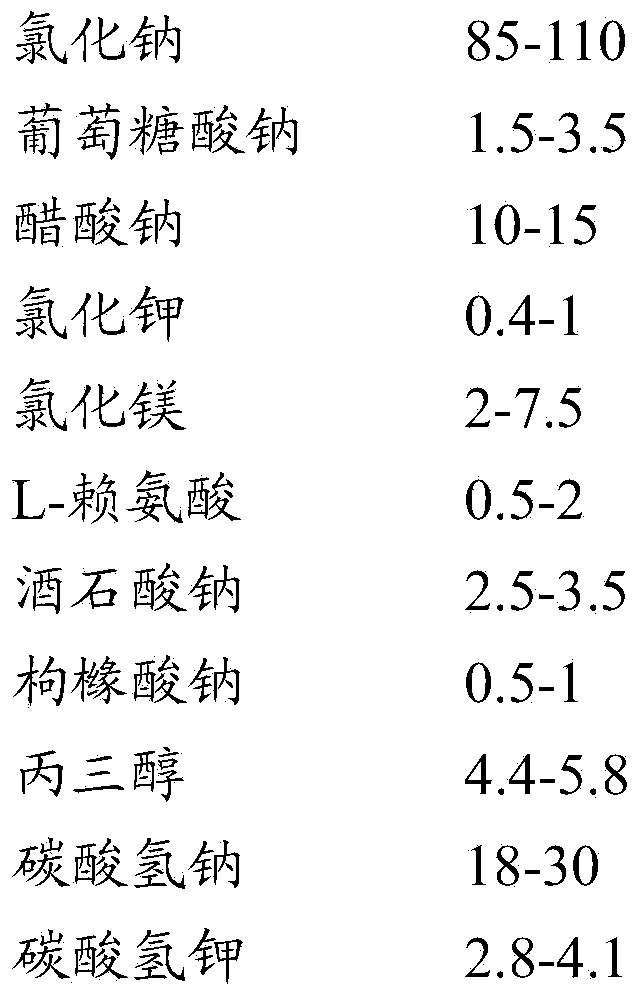
[0071] (1) Use an electronic balance to accurately weigh 95mmol sodium chloride, 1.5mmol sodium gluconate, 10mmol sodium acetate, 0.4mmol potassium chloride, 2.3mmol magnesium chloride, 0.5mmol L-lysine, 2.5mmol sodium tartrate, 0.6mmol citrate Sodium citrate, 4.5mmol glycerol, 26.7mmol sodium bicarbonate and 3.6mmol potassium bicarbonate;
[0072] (2) Add the above-mentioned weighed sodium chloride, sodium gluconate, sodium acetate, sodium tartrate, sodium citrate and sodium bicarbonate to water for injection, fully stir and dissolve to obtain the first dosing; wherein the amount of water for injection such that Na + Concentration 420mmol / L;
[0073] (3) Add the above-mentioned weighed potassium chloride, magnesium chloride, L-lysine, glycerol and potassium bicarbonate to the first dosing, fully stir and dissolve, then add water for injection to obtain the second dosing; The amount of water used is such that Na + Concentration 210mmol / L;
[0074] (4) Add activated carbon ...
Embodiment 2
[0078] (1) Use an electronic balance to accurately weigh 100mmol sodium chloride, 2.5mmol sodium gluconate, 15mmol sodium acetate, 0.607mmol potassium chloride, 3.4mmol magnesium chloride, 1mmol L-lysine, 3mmol sodium tartrate, 0.8mmol citric acid Sodium, 5mmol glycerol, 19.1mmol sodium bicarbonate and 3.893mmol potassium bicarbonate;
[0079] (2) Add the above-mentioned weighed sodium chloride, sodium gluconate, sodium acetate, sodium tartrate, sodium citrate and sodium bicarbonate to water for injection, fully stir and dissolve to obtain the first dosing; wherein the amount of water for injection such that Na + Concentration 435mmol / L;
[0080] (3) Add the above-mentioned weighed potassium chloride, magnesium chloride, L-lysine, glycerol and potassium bicarbonate to the first dosing, fully stir and dissolve, then add water for injection to obtain the second dosing; The amount of water used is such that Na + Concentration 290mmol / L;
[0081] (4) Add activated carbon for n...
Embodiment 3
[0085] (1) Use an electronic balance to accurately weigh 94.5mmol sodium chloride, 3.5mmol sodium gluconate, 15mmol sodium acetate, 0.5mmol potassium chloride, 4.224mmol magnesium chloride, 1.5mmol L-lysine, 3.5mmol sodium tartrate, 1mmol citrate Sodium citrate, 5.5mmol glycerol, 27mmol sodium bicarbonate and 4mmol potassium bicarbonate;
[0086] (2) Add the above-mentioned weighed sodium chloride, sodium gluconate, sodium acetate, sodium tartrate, sodium citrate and sodium bicarbonate to water for injection, fully stir and dissolve to obtain the first dosing; wherein the amount of water for injection such that Na + Concentration 450mmol / L;
[0087] (3) Add the above-mentioned weighed potassium chloride, magnesium chloride, L-lysine, glycerol and potassium bicarbonate to the first dosing, fully stir and dissolve, then add water for injection to obtain the second dosing; The amount of water used is such that Na + Concentration 375mmol / L;
[0088] (4) Add activated carbon fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com