Humanized anti-human interferon (IFN) alpha antibody and application thereof
An antibody and whole antibody technology, applied in the fields of application, antibody, anticytokine/lymphokine/interferon immunoglobulin, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1 Screening and Identification of Specific Human Anti-human Interferon Alpha Antibody
[0057] 1. Materials and methods:
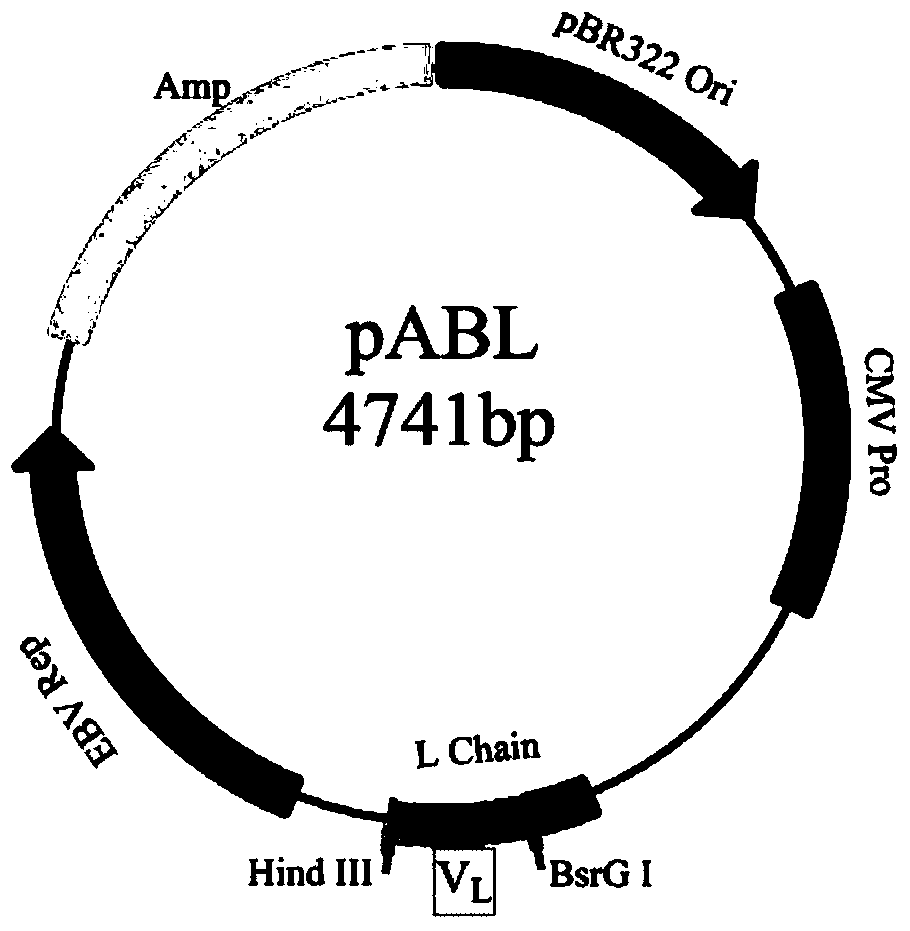
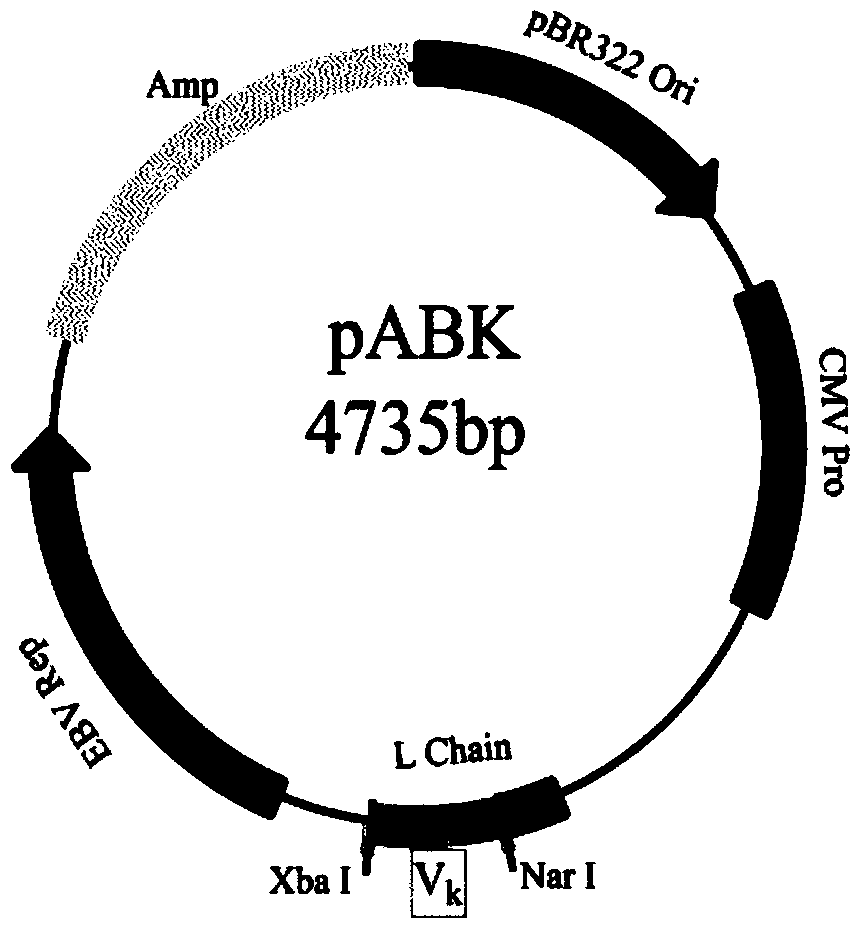
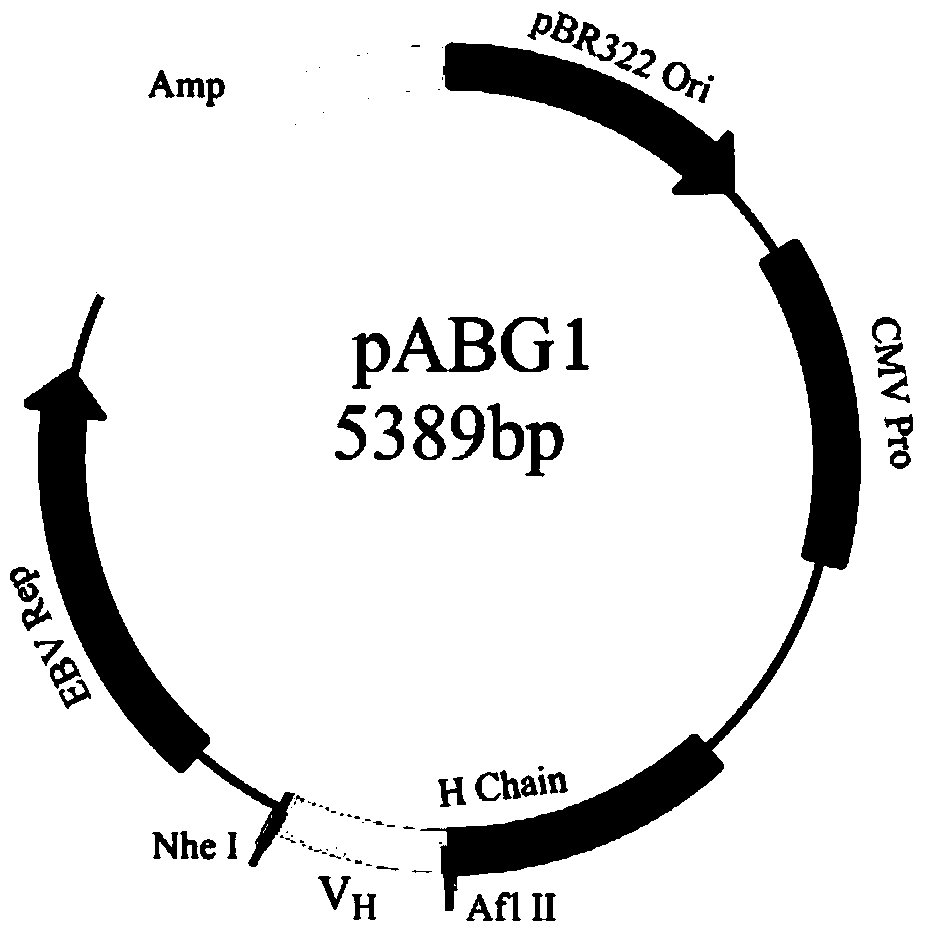
[0058]1. Materials: A large-capacity fully synthetic phage single-chain antibody library was constructed by the Academy of Military Medical Sciences of the Chinese People's Liberation Army (ZL200910091261.8), with a library capacity of 1.35×10 10 . Antigens for screening the antibody library were human IFNα_2b recombinantly expressed in Escherichia coli (ProSpec-Tany, Israel) and HSA-IFNα_1b and HSA-IFNα_2b fused with human serum albumin (HSA) (Antibody Online Company). The bacterial strain is XL1-Blue (Stratagene, USA); the phage used is M13KO7 (Invitrogene, USA). The eukaryotic expression vectors pABG1, pABK and pABL are preserved in our laboratory. The vector structure map is shown in Figure 1. Mammalian cells FreeStyle TM HEK293-T was purchased from Invitrogene. The variable region nucleic acid sequence synthesis service of MedImmune...
Embodiment 2
[0082] Example 2 Analysis of AIA22 affinity and neutralizing activity in vitro
[0083] 1. Materials and methods:
[0084] 1. Materials: CM5 chips were purchased from GE Healthcare Life Sciences. IFNα_1, IFNα_2, IFNα_4, IFNα_5, IFNα_6, IFNα_8, IFNα_10, IFNα_14, IFNα_16, IFNα_17, IFNα_21 and other subtypes were purchased from PBL Company (product number 11002-1). Daudi cells (human Burkitt's lymphoma cells) were purchased from the Cell Bank of the Type Culture Collection Committee of the Chinese Academy of Sciences. CCK-8 was purchased from Japan Dojin Chemical Co., Ltd. Neonatal bovine serum NCS was purchased from PAA Company. RPMI1640 cell culture medium was purchased from Gibco. Other related materials are the same as in Example 1.
[0085] 2. Method
[0086] 2.1 BIAcore3000 System Determination of Antibody Affinity
[0087] In this example, the BIAcore3000 system was used to detect the affinity of AIA22 to IFNα_2b and HSA-IFNα_1b. The specific steps are as follows: ...
Embodiment 3
[0116] Example 3 AIA22 light and heavy chain CDR region alanine scanning and mutant evaluation
[0117] 1. Materials and methods:
[0118] 1. Materials: Fortebio Octet system and Anti-Human IgG Fc Capture were purchased from iResearch Biotechnology (Shanghai) Co., Ltd. Other relates to material with embodiment 1, embodiment 2.
[0119] 2. Method
[0120] 2.1 Alanine scanning
[0121] Site-directed mutagenesis primers were designed to scan the 6 CDR regions of the AIA22 antibody for alanine, and if the position was alanine, it was mutated to Gly and Ser respectively. Methods The plasmid site-directed mutagenesis method was used, and the specific implementation method can refer to the literature [Wang Ronghao, Chen Ruichuan, Liu Runzhong. An optimized method for fast point mutations. Journal of Xiamen University (Natural Science Edition), 2008, Vol47, sup2, 282-285].
[0122] 2.2. ELISA detection of relative affinity
[0123] Antigens (HSA-IFNα_1b and HSA-IFNα_2b) were di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com