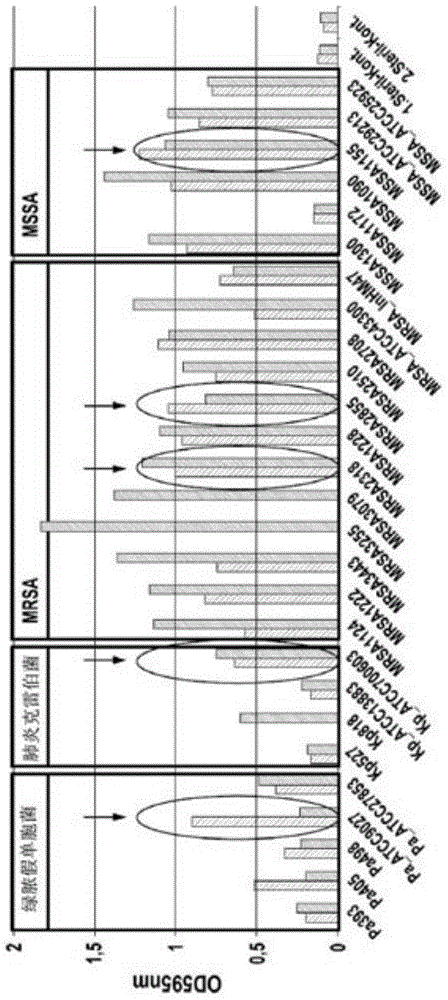
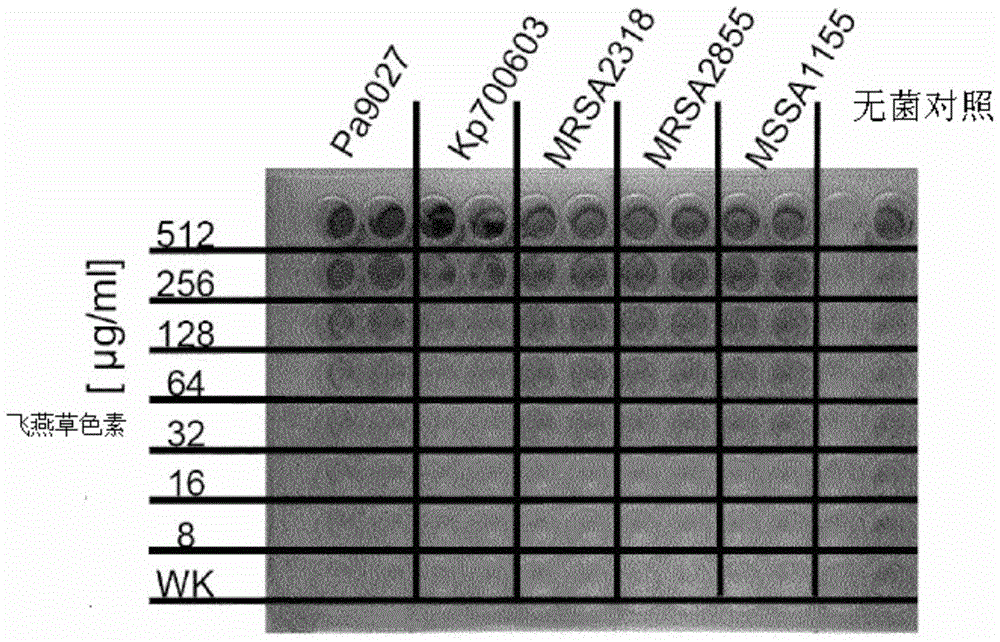
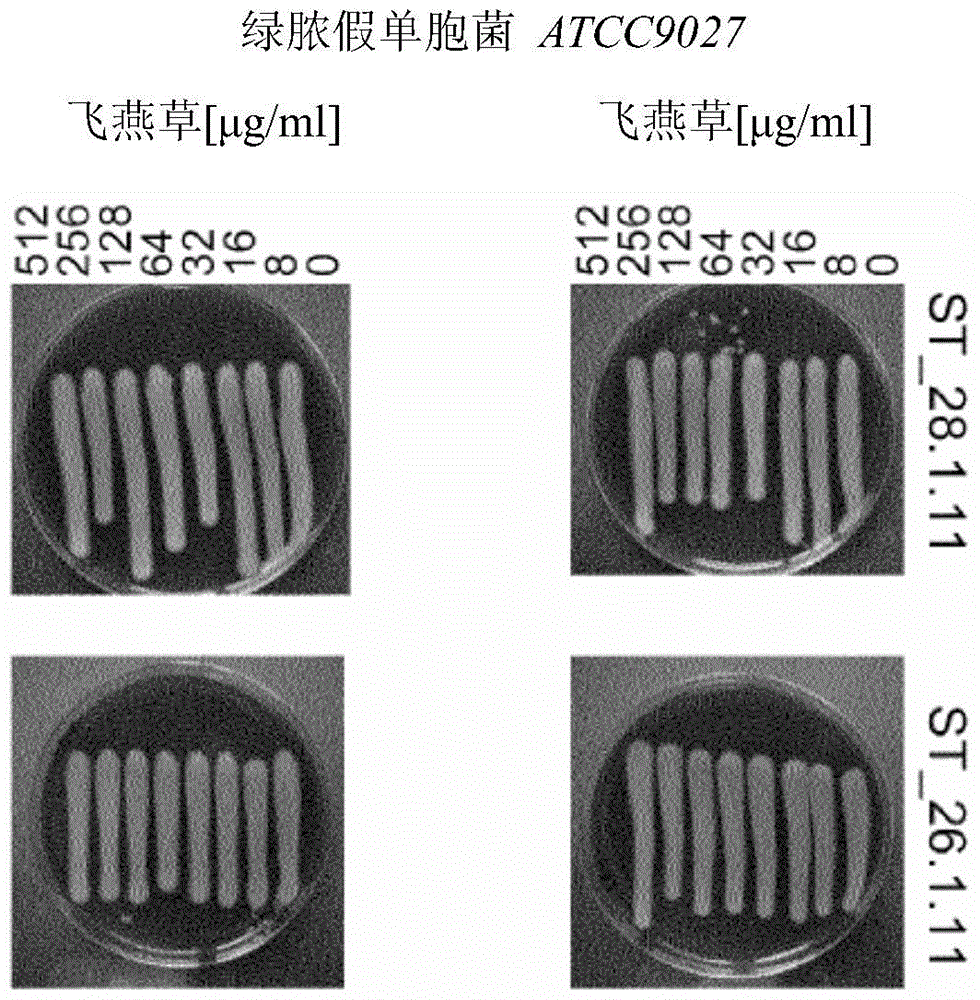
Application of Delphinidin against Staphylococcus aureus
A technology of delphinidin and staphylococcus, which is applied in the fields of application, antibacterial drugs, and medical preparations containing active ingredients, and can solve the problems of no longer having antibiotics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] Preparation of solutions and samples:
[0064] Dilute solution 1: Mixed solution of 100ml methanol and 2.6ml 1M hydrochloric acid
[0065] Dilute solution 2: 100ml 40% methanol and 2.6ml 1M hydrochloric acid mixed solution
[0066] Calibration solution: A reference solution of delphinidin was prepared as follows: 10 mg of delphinidin chloride was weighed into a 10 ml beaker and dissolved into dilution solution 1. After dissolving, it was further diluted about 10 times with Diluent 2 to prepare a solution with a concentration of about 0.1 mg / ml.
[0067] Control calibration solutions will be prepared in the same manner. The calibration solution must then be analyzed immediately by HPLC because delphinidin chloride is not stable in solution.
[0068] Prepare detection solution:
[0069] The delphinium content of the solid prepared in the present invention is determined according to the following scheme (see below for the preparation method): Weigh about 50 mg of the m...
Embodiment 1
[0072] Complex of delphinium and sulfobutyl ether-β-cyclodextrin (SBE-β-CD)
[0073] In this example, delphinidin was mixed with different cyclodextrins to observe the solubility of the complex in aqueous solution.
[0074] Usually, in the prepared neutral aqueous solution, the weight percent of each cyclodextrin is 10. However, when added to the aqueous solution of β-CD, the weight percentage of cyclodextrin is only 2 due to its poor solubility.
[0075] Each 5ml cyclodextrin aqueous solution was put into a glass beaker with corresponding purified water. Subsequently, an excess of delphinidin chloride was added. Wherein, the specific dosage of the excess delphinidin chloride is as follows: 10 mg in α-, β-, γ-cyclodextrin solution, HPBCD (2 hydroxypropyl-β-cyclodextrin) and SBE-β-cyclodextrin 15mg in CD.
[0076] The suspension was stirred for 20 hours at 30°C in the dark. Subsequently, the suspension was filtered using a filter membrane with a pore size of 0.22 μm.
[0...
Embodiment 2
[0081] Effect of pH
[0082] In this example, the effect of pH on the solubility of delphinidin-SBE-β-CD in aqueous solution will be investigated. The SBE-β-CD aqueous solution was prepared according to the method described in Example 1, and then the solution was adjusted to the acidic pH values shown in Table 2 using 1M hydrochloric acid. Subsequently, delphinidin chloride was added to the aqueous solution and further processed according to the method described in Example 1, with the only difference that the stirring time was limited to 2.5 hours. The final results are shown in Table 2.
[0083] pH Delphinidin Chloride 6.0 0.60mg / ml 4.8 2.12mg / ml 4.1 2.03mg / ml
[0084] It can be seen that the solubility of the delphinidin complex in the pH range of 4 to 5 is about 3 times that of the delphinidin complex at neutral pH.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com