Process method of rare earth oxysulfide by using alkali chloride
A technology of rare earth sulfur oxides and alkali metal chlorides, applied in rare earth metal compounds, rare earth metal sulfides, chemical instruments and methods, etc., can solve the problems of large particles, reduced luminescence performance, etc., and achieves simple preparation and synthesis methods. Production cost, effect of reducing particle distribution range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
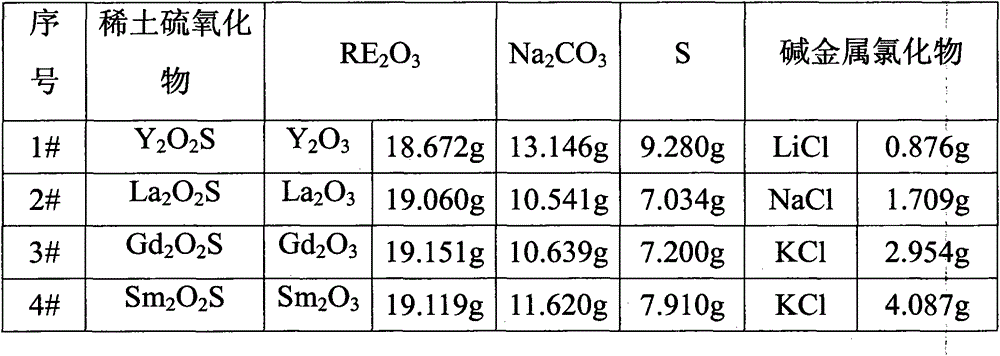
[0020] Raw material composition is as in table 1 1 # Shown, specific preparation method comprises the following steps:
[0021] ① Weigh 18.672g Y by weight 2 o 3 , 13.146g Na 2 CO 3 (mol(Na 2 CO 3 ):mol(Y 2 o 3 )=1.5), 9.280g sublimation sulfur (mol (sublimation sulfur): mol (Y 2 o 3 )=3.5) and 0.876gLiCl(mol(LiCl):mol(Y 2 o 3 )=0.25), mix the weighed materials evenly;
[0022] ②Put the uniformly mixed material into a crucible with a cover inside and outside, fill the gap between the two crucibles with high-temperature resistant powder and compact it;
[0023] ③Raise the temperature of the crucible containing the mixture to 300°C at a heating rate of 2°C / min, and keep it warm for 1h, then raise the temperature to 1100°C at a heating rate of 3°C / min, sinter for 3h, and then cool down to room temperature with the furnace;
[0024] ④ The sintered product is washed with hot water and stirred, and suction filtered;
[0025] ⑤Put the washed product into a constant temp...
Embodiment 2
[0029] ① Weigh 19.060g La by weight 2 o 3 , 10.541g Na 2 CO 3 (mol(Na 2 CO 3 ): mol (La 2 o 3 )=1.7), 7.034g sublimation sulfur (mol (sublimation sulfur): mol (La 2 o 3 )=3.75) and 1.709gNaCl(mol(NaCl):mol(La 2 o 3 )=0.5), mix the weighed materials evenly;
[0030] ②Put the uniformly mixed material into a crucible with a cover inside and outside, fill the gap between the two crucibles with high-temperature resistant powder and compact it;
[0031] ③Raise the temperature of the crucible containing the mixture to 330°C at a heating rate of 2.5°C / min, and keep it warm for 1.5h, then raise the temperature to 1150°C at a heating rate of 3.5°C / min, sinter for 4h, and then cool down to room temperature with the furnace;
[0032] ④ The sintered product is washed with hot water and stirred, and suction filtered;
[0033] ⑤Put the washed product into a constant temperature blast drying oven to dry to obtain rare earth sulfur oxides.
[0034] The test results of this rare ea...
Embodiment 3
[0037] Composition as in Table 1 3 # Shown, specific preparation method comprises the following steps:
[0038] ①Weigh 19.151gGd by weight 2 o 3 , 10.639g Na 2 CO 3 (mol(Na 2 CO 3 ): mol (Gd 2 o 3 )=1.9), 7.200g sublimated sulfur (mol (sublimed sulfur): mol (Gd 2 o 3 )=4.25) and 2.954gKCl(mol(KCl):mol(Gd 2 o 3 )=0.75), mix the weighed materials evenly;
[0039] ②Put the uniformly mixed material into a crucible with a cover inside and outside, fill the gap between the two crucibles with high-temperature resistant powder and compact it;
[0040] ③Raise the temperature of the crucible containing the mixture to 360°C at a heating rate of 3°C / min, and keep it warm for 2h, then raise the temperature to 1200°C at a heating rate of 4°C / min, sinter for 5h, and then cool down to room temperature with the furnace;
[0041] ④ The sintered product is washed with hot water and stirred, and suction filtered;
[0042] ⑤Put the washed product into a constant temperature blast dry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com