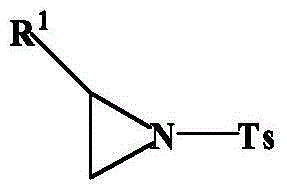
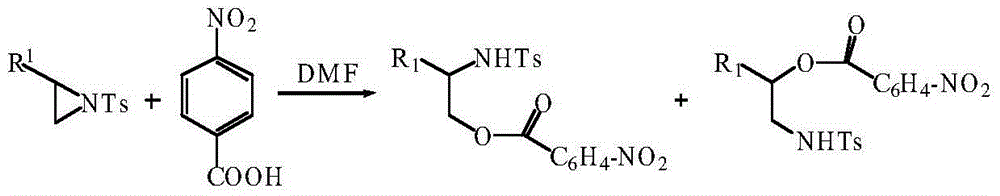
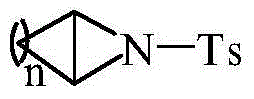
Method using carboxylic acid for ring opening of aziridine compound
An aziridine and carboxylic acid compound technology, applied in the field of organic synthesis, can solve the problems of low selectivity, few reports, low regioselectivity, etc., and achieve the effects of high yield, wide applicability, and environmental protection of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Add 0.2mmol of aziridine with the structural formula 1a in Table 1, 0.24mmol of p-nitrobenzoic acid, and 2.5mL of N,N-dimethylformamide into the test tube, heat to 70°C and stir the reaction for 35h, add K2 CO 3 The remaining acid was removed from the solution, and the crude product was obtained by extraction, washing and drying. The crude product was purified by silica gel chromatography to obtain a ring-opened product with a single configuration. The structural formula is shown in 3a in Table 1. The product was characterized by nuclear magnetic resonance to confirm the structure of the product.
[0046] Table 1 Reaction of aziridine 1a with p-nitrobenzoic acid
[0047]
[0048] 3a: white solid; mp 164-166°C; 1 H NMR (600MHz, CDCl 3 ):δ2.17(s,3H),2.39(d,J=1.8Hz,3H),3.39-3.50(m,1H),4.41-4.44(m,1H),5.27-5.30(t,J=7.2 Hz,1H),6.19-6.21(dd,J=9.0,4.2Hz,1H),7.02-7.03(d,J=8.4Hz,1H),7.07-7.08(d,J=7.2Hz,1H),7.13 -7.24(m,3H),7.29-7.30(d,J=7.8Hz,1H),7.52-7.53(m,1H),7.70-7.7...
Embodiment 2
[0056] Add 0.2mmol of aziridine with the structural formula 1b in Table 3, 0.24mmol of p-nitrobenzoic acid, and 1.5mL of N,N-dimethylformamide into the test tube, heat to 55°C and stir the reaction for 39h, add K 2 CO 3 The residual acid was removed from the solution, and the crude product was obtained by extraction, washing and drying. The crude product was purified by silica gel chromatography to obtain a ring-opened product with a single configuration. The structural formula is shown in 3b in Table 3. The product was characterized by nuclear magnetic resonance. The structure of the product.
[0057] Table 3 Reaction of aziridine 1b with p-nitrobenzoic acid
[0058]
[0059] 3b: white solid; mp 140-142°C; 1 H NMR (600MHz, CDCl 3 ):δ2.33(s,3H),2.40(s,3H),3.44-3.53(m,1H),4.43-4.54(m,1H),5.19-5.21(m,1H),5.96-5.98(dd ,J=7.8,4.2Hz,1H),7.05-7.08(m,2H),7.14-7.15(d,J=7.8Hz,2H),7.20-7.24(dd,J=12.0,8.4Hz,2H), 7.57-7.58(d, J=8.4Hz, 1H), 7.68-7.70(d, J=8.4Hz, 1H), 8.06-8.07(d, ...
Embodiment 3
[0067] Add 1mmol of aziridine, 1.1mmol of p-nitrobenzoic acid, and 10mL of N,N-dimethylformamide into a 50mL round-bottomed flask, and heat to 75°C and stir for 30h. Join K 2 CO 3 The residual acid was removed from the solution, and the crude product was obtained after extraction, washing and drying. The crude product was purified by silica gel chromatography to obtain a ring-opened product with a single configuration. The structural formula is shown in 3c in Table 5. The product was characterized by H NMR The structure of the product was confirmed.
[0068] Table 5 Reaction of aziridine 1c with p-nitrobenzoic acid
[0069]
[0070] 3c: white solid; mp 146-148°C; 1 H NMR (600MHz, CDCl 3 ):δ2.31(s,3H),3.44-3.49(m,1H),4.42-4.49(m,1H),4.45-4.76(td,J=7.8,1.8Hz,1H),6.17-6.19(d ,J=7.8Hz,1H),7.06-7.07(d,J=7.8Hz,1H),7.09-7.7.14(m,2H),7.17-7.18(m,1H),7.23-7.24(d,J =7.8Hz,1H),7.27-7.28(m,1H),7.56-7.57(m,1H),7.69-7.70(dd,J=7.8,6.0Hz,1H),8.04-8.20(m,4H)ppm .
[0071] The compar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com