Silicon-containing bismaleimide resin and preparation thereof
A technology of bismaleimide resin and bismaleimide, which is applied in the field of high-performance polymer material manufacturing and can solve problems such as complicated synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0148] A kind of preparation method of modified bismaleimide resin, described method comprises steps:
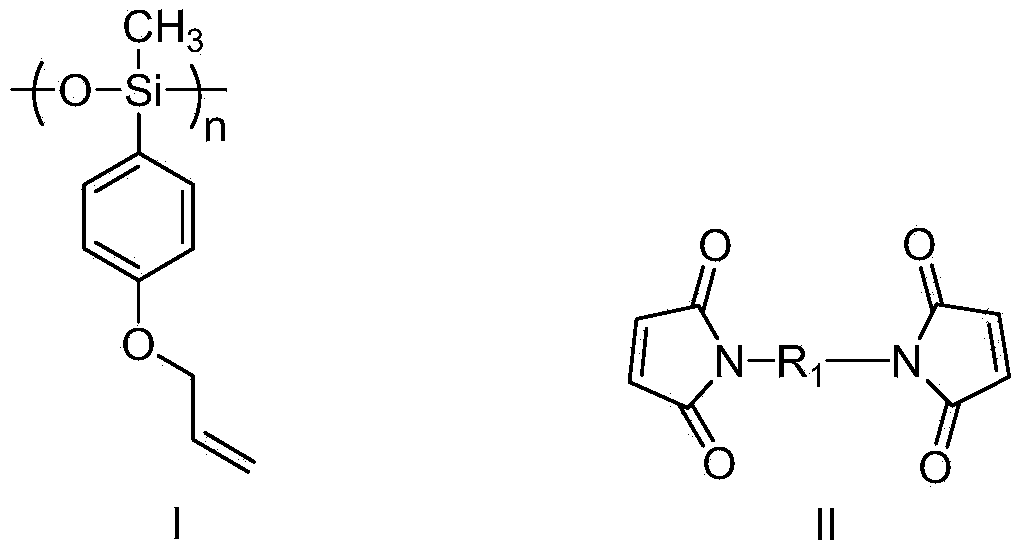
[0149] Copolymerize with allyl substituted aryl siloxane such as formula I and bismaleimide such as formula II to obtain modified bismaleimide resin:
[0150]
[0151] In the formula, n≥2, preferably, n=10~20;
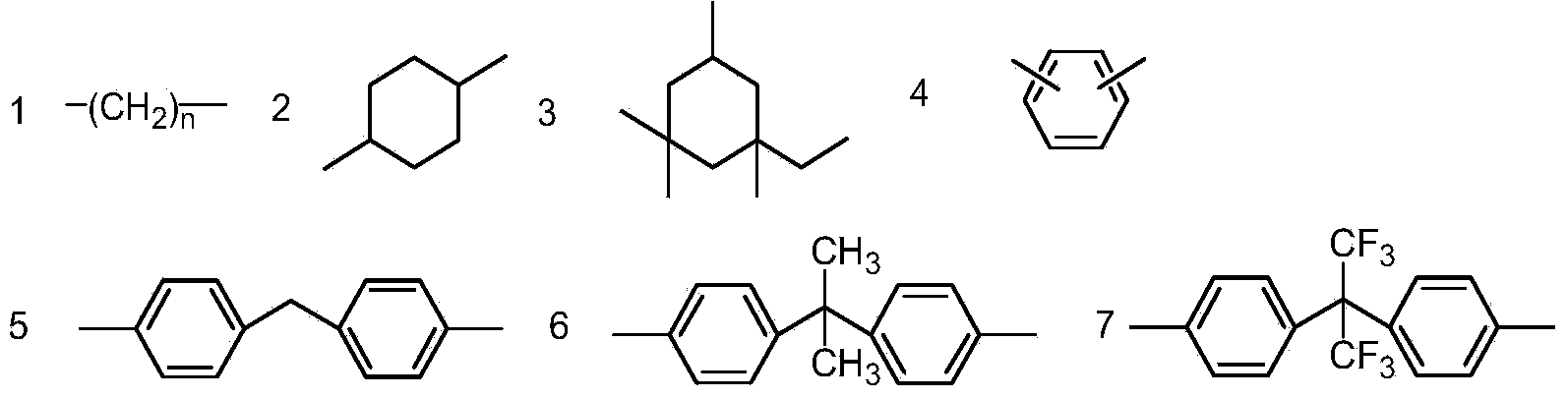
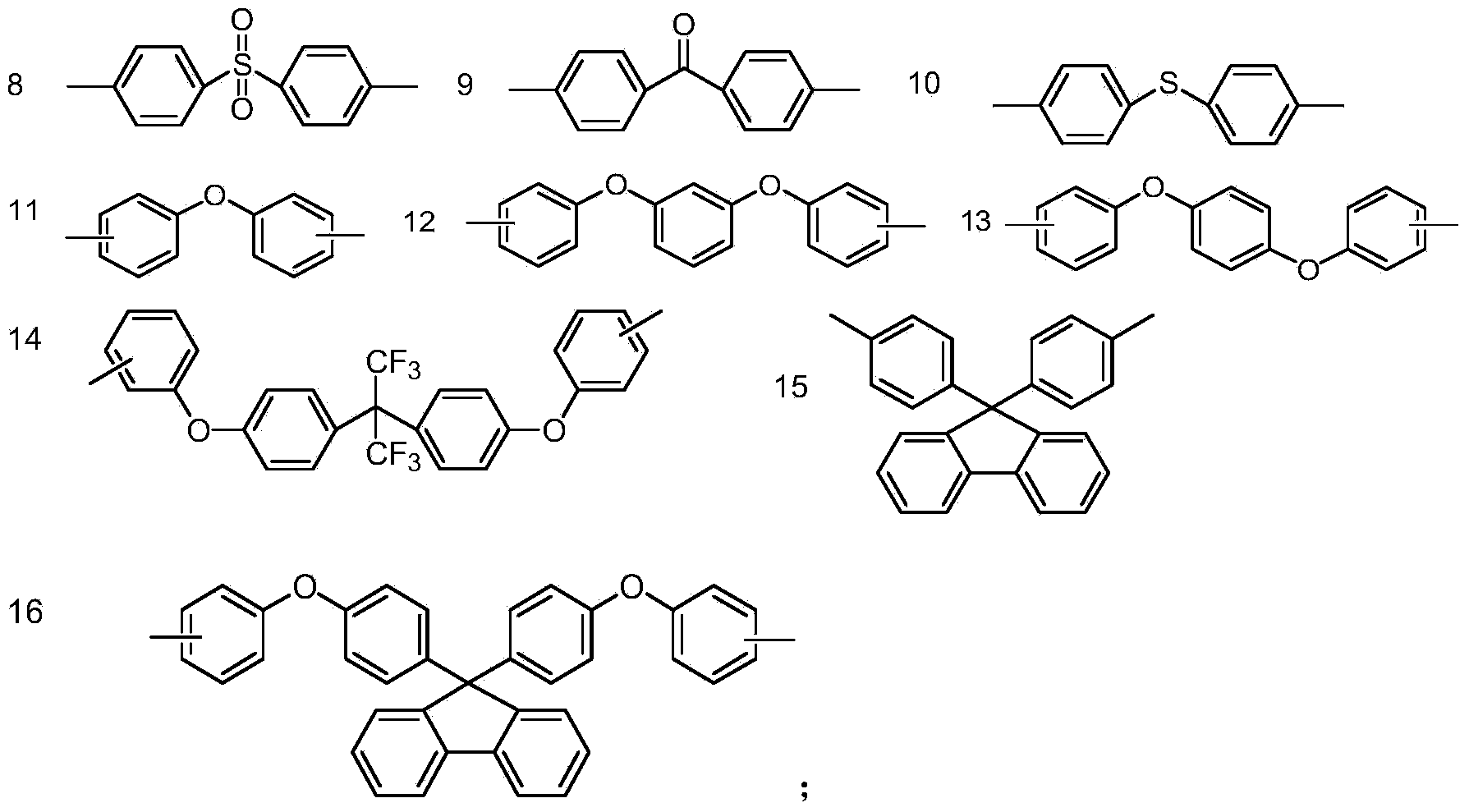
[0152] R 1 Selected from the group consisting of substituted or unsubstituted C1-C30 alkyl, substituted or unsubstituted C1-C30 aryl, substituted or unsubstituted C2-C30 aryl-alkyl, substituted or unsubstituted C3 -C30 aryl-alkyl-aryl, substituted or unsubstituted C3-C30 aryl-carbonyl-aryl, substituted or unsubstituted C2-C30 aryl-oxy-aryl, substituted or unsubstituted C2-C30 aryl-oxygen-aryl-oxygen-aryl, substituted or unsubstituted C2-C30 aryl-sulfur-aryl, substituted or unsubstituted C2-C30 aryl-sulfone- Aryl;
[0153] Wherein, the substitution refers to being substituted by one or more groups selected from the group consisting of halogen, C1-C4 alkyl, C1-C...
Embodiment 1
[0171] The synthesis of embodiment 1 p-bromoallyl phenyl ether
[0172]
[0173] Under argon protection, add 51.9 grams of 4-bromophenol (0.3mol), 60.5 grams of allyl bromide (0.5mol) and 300 milliliters of DMSO (dimethyl sulfoxide) steamed now in the reaction apparatus, stir at room temperature for 30 Add 53 grams of anhydrous sodium carbonate (0.5mol) after 10 minutes, react at room temperature for 10 hours, pour the reaction mixture solution into water, stir vigorously for 20 minutes, extract the product in batches with chloroform, and extract the solution with saturated sodium chloride aqueous solution After washing, the chloroform was removed by rotary evaporation, and the obtained solid was washed with water and a small amount of ethanol to obtain 100 g of the product with a yield of 94%. Hydrogen spectrum characterization ( 1 H NMR, 300MHz, CDCl 3 ) 7.55 (d, 2H), 7.02 (d, 2H), 5.94 (d, 1H), 5.33 (d, 1H), 5.22 (d, 1H), 4.66 (s, 2H).
Embodiment 2
[0174] The synthesis of embodiment 2 p-bromoallyl phenyl ethers
[0175]
[0176] Under argon protection, add 51.9 grams of 4-bromophenol (0.3mol), 38 grams of allyl chloride (0.5mol) and 300 milliliters of DMSO (dimethyl sulfoxide) steamed now in the reaction apparatus, stir at room temperature for 30 Add 53 grams of anhydrous sodium carbonate (0.5mol) after 10 minutes, react at room temperature after 10 hours, pour the reaction mixed solution into water, stir vigorously for 20 minutes, the product is extracted with chloroform in batches, and the extract is saturated sodium chloride aqueous solution After washing, the chloroform was removed by rotary evaporation, and the obtained solid was washed with water and a small amount of ethanol to obtain 88 g of the product, with a yield of 83%. Hydrogen spectrum characterization ( 1 H NMR, 300MHz, CDCl 3 ) 7.55 (d, 2H), 7.02 (d, 2H), 5.94 (d, 1H), 5.33 (d, 1H), 5.22 (d, 1H), 4.66 (s, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com