Method for separating human chorionic gonadotropin monoclonal antibody segment
A technology of chorionic gonadotropin and monoclonal antibody, which is applied in the direction of immobilization on/in it, fermentation, etc., and can solve the problem of reduced catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
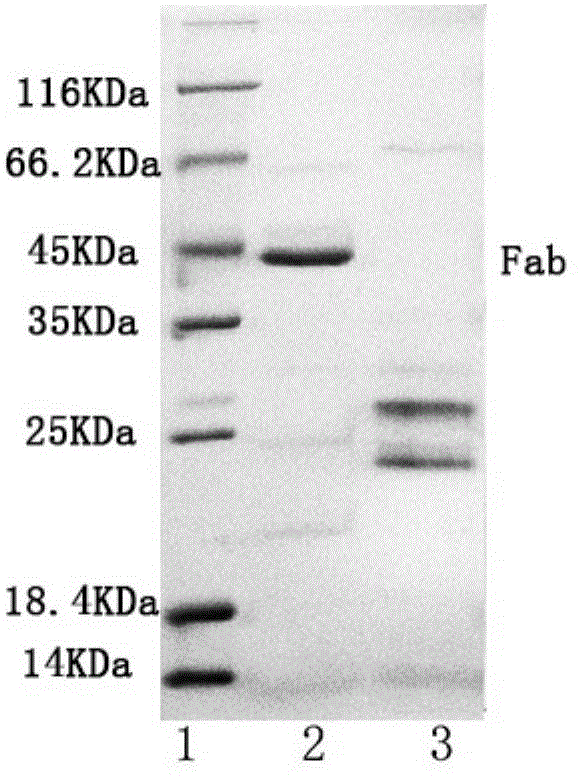
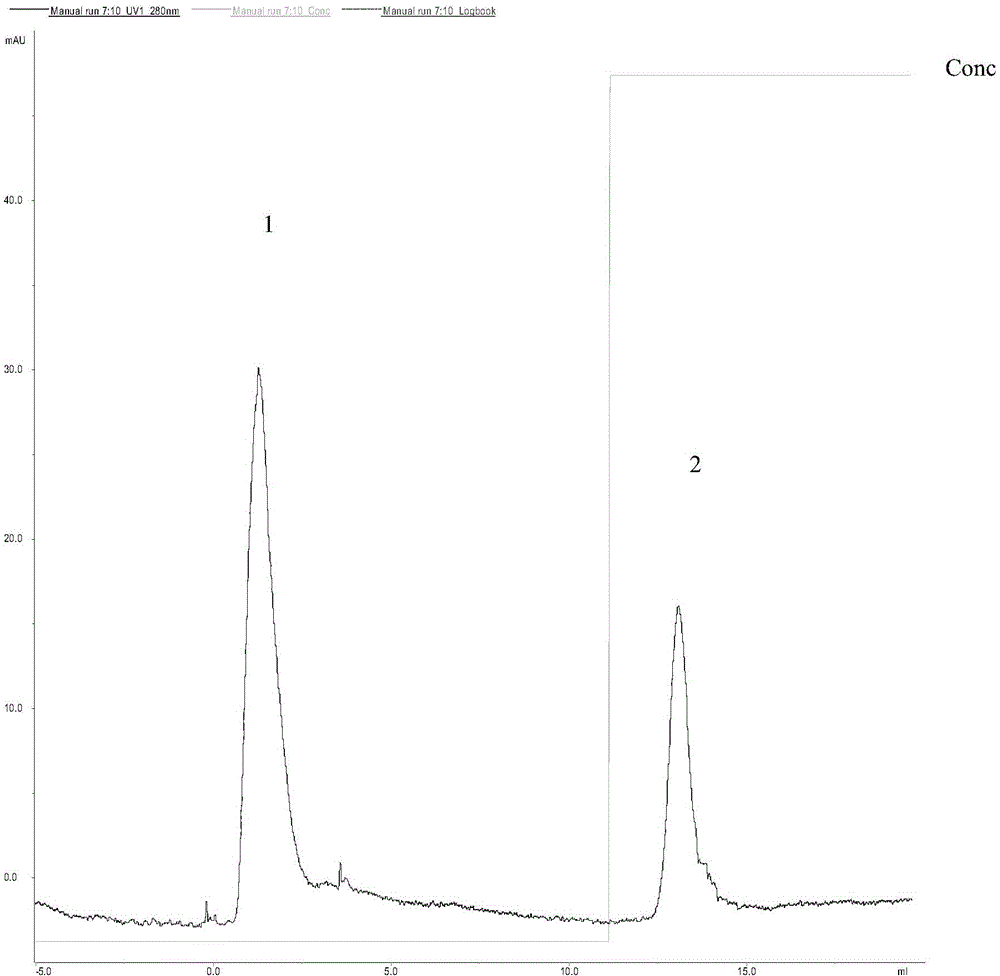
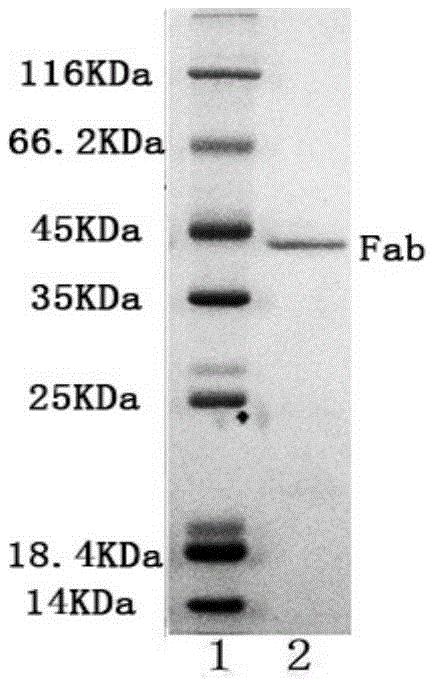
[0029] 1) Immobilization of papain: Weigh 10 mg of NHS-PNIPAM and dissolve it in 100 μL of N,N-dimethylformamide that has been dried overnight with activated 4A molecular sieves (that is, 4A molecular sieves are activated at 300 ° C for 4 hours, and then added after cooling In N,N-dimethylformamide, separate layers overnight, take the upper layer), add 500μL, 2mg / ml papain solution (prepared with boric acid buffer solution with pH 8.5, 0.1mol / L, the specific enzyme activity of papain 570.3U / mg), shake in a water bath at 10°C for 2h, dilute the resulting reaction solution with pH 7.0, 0.05mol / L PBS buffer solution to 4 times the volume, centrifuge at 11000r / min at 40°C for 30min, collect the precipitate, and use a 4°C pH 7.0, dissolved in 0.05mol / L phosphate buffer, after the precipitate was completely dissolved, centrifuged at 40°C, 11000r / min for 30min, this process was repeated 3 times (i.e. dissolved, centrifuged 3 times), and the precipitate was collected for immobilization...
Embodiment 2
[0042] 1) Immobilization of papain: Weigh 10 mg of NHS-PNIPAM and dissolve it in 100 μL of N,N-dimethylformamide dried overnight with activated 4A molecular sieve (the method is the same as in Example 1), add 500 μL, 20 mg / ml Papain solution (prepared with pH 8.5, 0.1mol / L boric acid buffer solution, 570.3U / mg), shake in a water bath at 25°C for 10h, and dilute the resulting reaction solution to 4 times with pH 7.0, 0.05mol / L PBS buffer solution Volume, centrifuge at 11000r / min at 40°C for 30min, collect the precipitate, dissolve it with pH 7.0, 0.05mol / L phosphate buffer at 4°C, after the precipitate is completely dissolved, centrifuge at 11000r / min at 40°C for 30min, repeat the process 3 times (dissolving and centrifugation repeated 3 times), the collected precipitate is immobilized papain (78.5U / mg carrier, specific enzyme activity 245.3U / mg), and finally dissolved in the newly prepared pH 7.0, 0.05mol / L Store in phosphate buffer at 4°C to obtain immobilized papain solution...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Absorb light | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com