Treponema pallidum specific antibody chemical light emitting detection kit and preparation method thereof
A technology for chemiluminescence detection and treponema pallidum, which is applied in the direction of chemiluminescence/bioluminescence, analysis by making materials undergo chemical reactions, and measurement devices, can solve the problems of inability to culture Treponema pallidum in vitro, high false positive rate of cardiolipin antibodies, inspection The positive rate is not high, and the detection time is shortened, the sensitivity is improved, and the precision is improved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
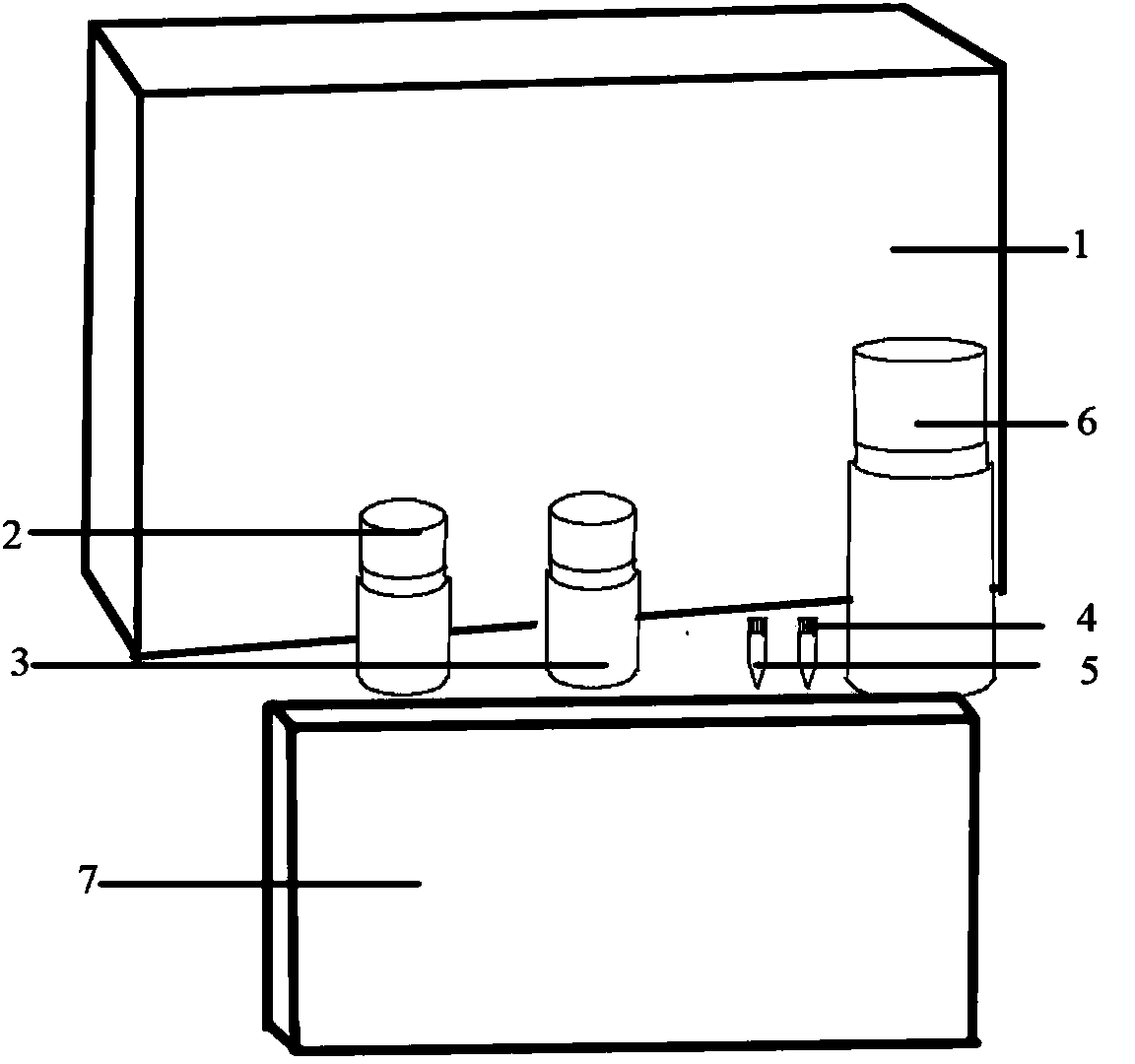
[0029] see figure 1 , the Treponema pallidum-specific antibody chemiluminescence detection kit is provided with an outer packing box 1, an alkaline phosphatase-labeled recombinant antigen bottle 2, a luminescent substrate bottle 3, a Treponema pallidum-specific antibody negative control bottle 4, and a Treponema pallidum-specific antibody bottle. 5 bottles of positive control substance, 6 bottles of washing solution, and 7 bottles of recombinant antigen-coated microwell plate; 2 bottles of alkaline phosphatase-labeled recombinant antigen, 3 bottles of luminescent substrate, 4 bottles of negative control substance specific antibody against Treponema pallidum, Treponema pallidum The specific positive control bottle 5, the washing solution bottle 6 and the recombinant antigen-coated microwell plate 7 are arranged in the outer packaging box 1; the alkaline phosphatase-labeled recombinant antigen bottle 2 is equipped with alkaline phosphatase-labeled recombinant antigen and luminesc...
Embodiment 2
[0048] The following shows the use of the Treponema pallidum antibody high-throughput detection kit to detect Treponema pallidum-specific antibodies in clinical specimens of patients:
[0049]1. Specimen processing: Serum: 5mL of venous blood, placed in a 37°C water bath for 30 minutes, centrifuged at 3000g for 10 minutes, and the supernatant was used as a test sample for later use.
[0050] 2. Adding samples: add 100 μL of samples to the reaction plate, and make blank, negative and positive control wells at the same time. Incubate at 37°C for 1h.
[0051] 3. Washing: After reacting at 37°C for 30 minutes, the measured syphilis-specific antibody binds to the syphilis-specific recombinant antigen coated on the microwell plate, and the unbound free components are separated by washing;
[0052] 4. Add alkaline phosphatase to label the recombinant antigen, then add luminescent substrate working solution, alkaline phosphatase catalyzes dephosphorylation of the substrate, and emits...
Embodiment 3
[0054] The performance test of the Treponema pallidum antibody high-throughput detection kit is given below.
[0055] (1) Conformity rate of positive samples
[0056] Use 50 positive reference sera of syphilis-specific antibodies to test, and calculate the positive coincidence rate.
[0057] (2) Negative specimen coincidence rate
[0058] Use 50 samples of syphilis-specific antibody-negative reference sera to test, and calculate the negative coincidence rate.
[0059] (3) Intra-batch variance
[0060] The same batch of kits is tested with characteristic serum, requiring CV ≤ 10%.
[0061] (4) Difference between batches
[0062] Different batches of kits are tested with characteristic serum, requiring CV ≤ 12%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com