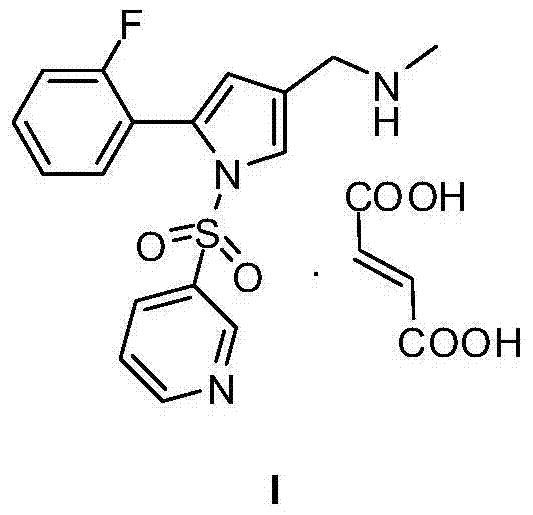
Crystal form of fumarate of pyrrole derivative
A fumarate and crystallization technology, applied in the direction of organic chemistry, etc., to achieve the effect of high purity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
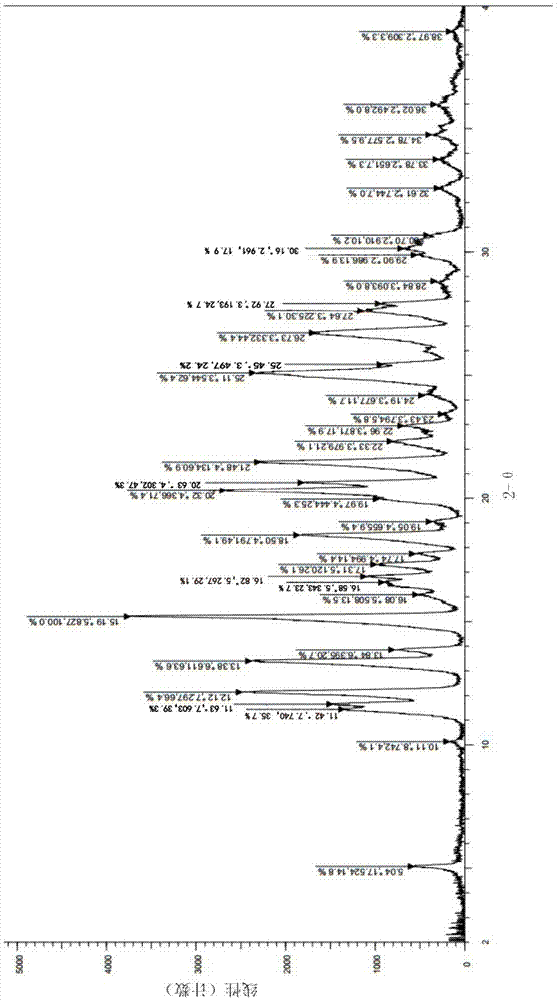
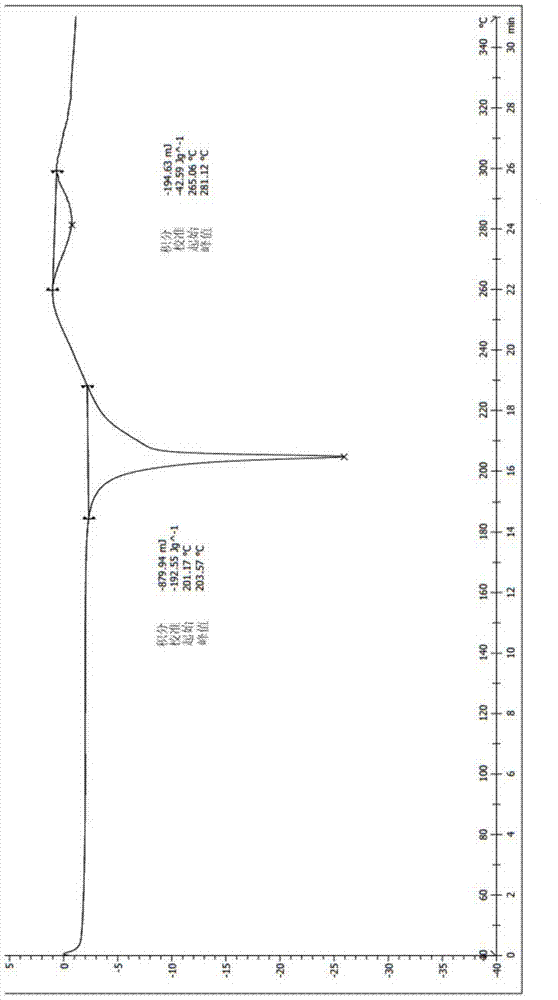
Image
Examples
Embodiment 1
[0042] The preparation method of pyrrole derivative maleate with crystal form A is described in detail below.
[0043] Step 1: Synthesis of 5-(2-fluorophenyl)-1-(pyridine-3-sulfonyl)-1H-pyrrole-3-carbaldehyde
[0044] Compound II (260 mg) was dissolved in tetrahydrofuran (50 ml), 60% NaH was added, and the reaction was stirred at room temperature for 30 minutes. Then add 15-crown-5 (1.5g), stir at room temperature for 1 hour, then add pyridine-3-sulfonyl chloride, stir at room temperature for 2 hours until the reaction is complete by TLC, then add 20 mL of saturated brine to the reaction system , extracted with ethyl acetate (100mL×2), combined the organic phases, washed the organic phase with 50ml of saturated brine, dried with an appropriate amount of anhydrous sodium sulfate, filtered, and concentrated the filtrate under reduced pressure to obtain the crude compound IV (200mg) which was directly used in the next reaction.
[0045] Step 2: Synthesis of 1-[5-(2-fluorophenyl)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com