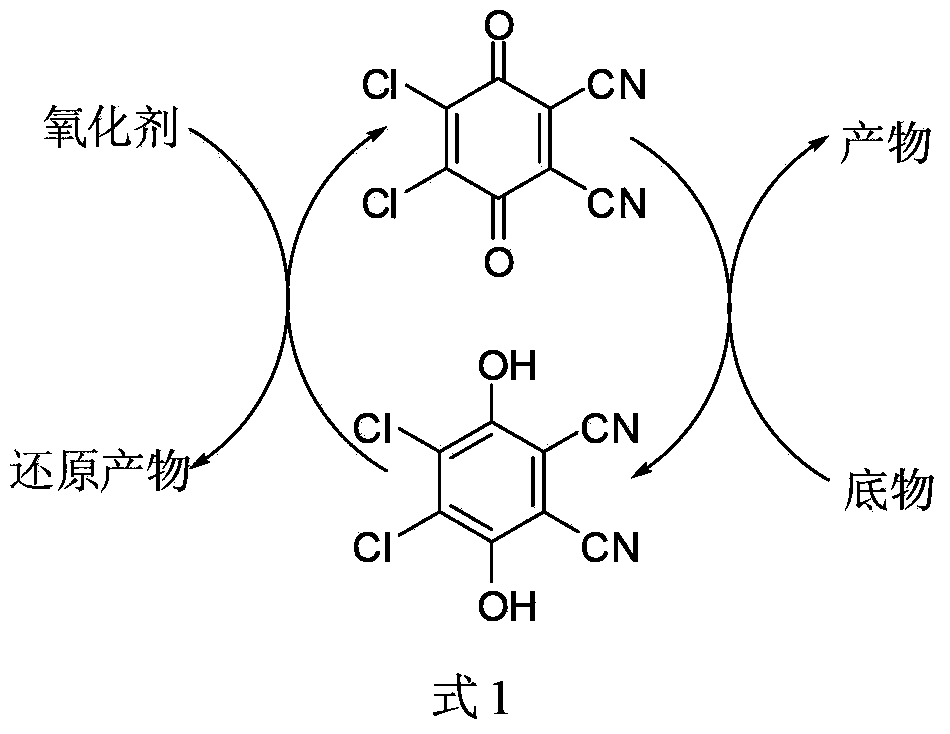
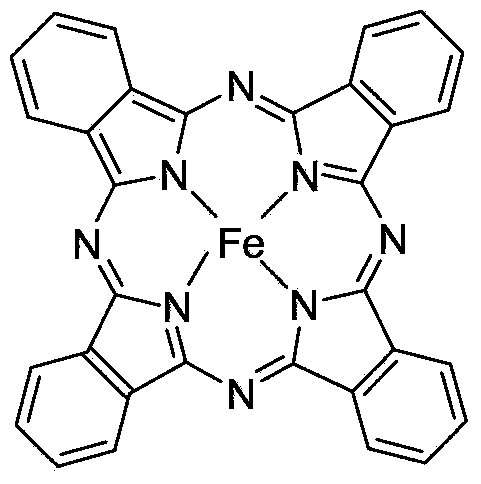
Method for preparing aldehyde and ketone through alcohol oxidation
A technology of alcohol oxidation and oxygen, which is applied in the direction of chemical instruments and methods, carbon-based compound preparation, organic compound preparation, etc., can solve problems such as pollution and post-processing troubles, and achieve the effects of environmental friendliness, easy product, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
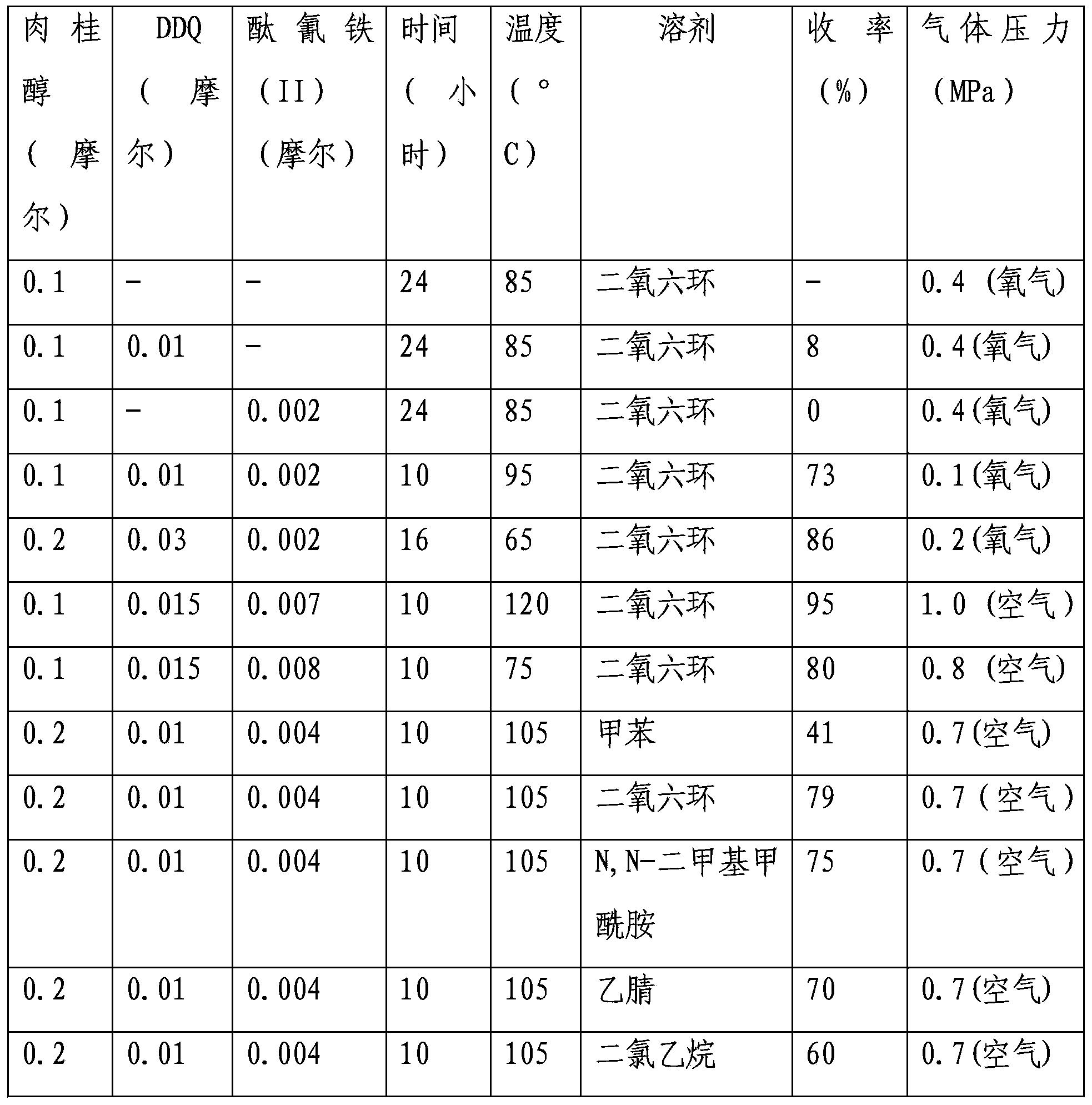
[0018] Example 1. Investigation of reaction conditions
[0019] First, we used cinnamyl alcohol as a model substrate to investigate the reaction conditions, and the product was cinnamaldehyde. 1 HNMR(300MHz, CDCl 3 )δ9.71(d,J=7.8Hz,1H), 7.60-7.52(m,3H), 7.47-7.42(m,3H), 6.75(dd,J=15.9,7.8Hz,1H), 13 C NMR(100.6MHz, CDCl 3 )δ128.2, 128.3, 128.8, 131.0, 133.7, 152.7, 193.6.
[0020] The typical reaction steps are as follows: add 13.4g (0.1mol) cinnamyl alcohol, 2.27g (0.01mol) DDQ, 1.137g ferric phthalocyanine (II), 30mL dioxane into a 100mL stainless steel reactor, seal it, and exchange oxygen 3 Next, the oxygen pressure was 0.1Mpa, and the mixture was stirred at 95°C for 10 hours. The dioxane was removed under reduced pressure, the resulting mixture was dissolved in dichloromethane, washed with water, dried to remove the dichloromethane, and 9.7 g of cinnamaldehyde was obtained by column separation (yield 73%).
[0021] The results are shown in Table 1
[0022] Table 1 Investigation o...
Embodiment 2
[0025] Add 124.2g p-hydroxybenzyl alcohol, 22.7g DDQ, 22.73g ferric phthalocyanine (II), 340mL N,N-dimethylformamide into a 500mL reaction flask, seal it, exchange oxygen 3 times, connect the oxygen balloon (0.1Mpa ), stirring at 100°C for 13h. The N,N-dimethylformamide was removed under reduced pressure, the resulting mixture was dissolved in dichloromethane, washed with water, dried to remove the dichloromethane, and 100 g (yield: 81%) of p-hydroxybenzaldehyde light yellow crystals were obtained by column separation. 1 H NMR(400MHz,DMSO):δ6.93(d, 3 J H,H =8.8Hz,1H),7.76(d, 3 J H,H =8.8Hz,2H),9.78(s,1H),10.58(s,1H); 13 C NMR (100.6 MHz, DMSO) δ 116.0, 128.6, 132.3, 163.5, 191.1.
Embodiment 3
[0026] Example 3, benzyl alcohol oxidation
[0027] Add 10.8g of benzyl alcohol, 3.4g of DDQ, 4.25g of phthalocyanine iron(II), and 50mL of acetonitrile into a 100mL stainless steel reactor, and react at 95°C under 0.3MPa oxygen pressure to cool to room temperature for 16 hours, and slowly release oxygen. The acetonitrile was removed by pressure, the resulting mixture was dissolved in dichloromethane, washed with water, dried to remove the dichloromethane, and 9 g (yield: 84%) of benzaldehyde was obtained by column separation. 1 H NMR(300MHz, CDCl 3 ,): δ7.51(t,J=7.6Hz,2H), 7.61(t,J=7.2Hz,1H), 7.86(d,J=7.6Hz,2H), 9.99(s,1H). 13 C NMR(100.6MHz, CDCl 3 ): δ128.7, 129.4, 134.1, 136.0, 192.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com