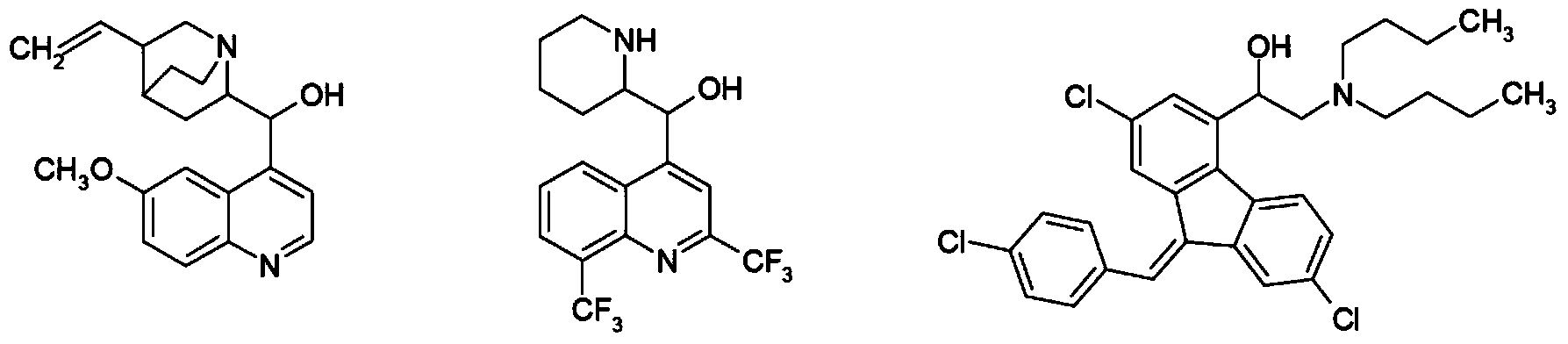
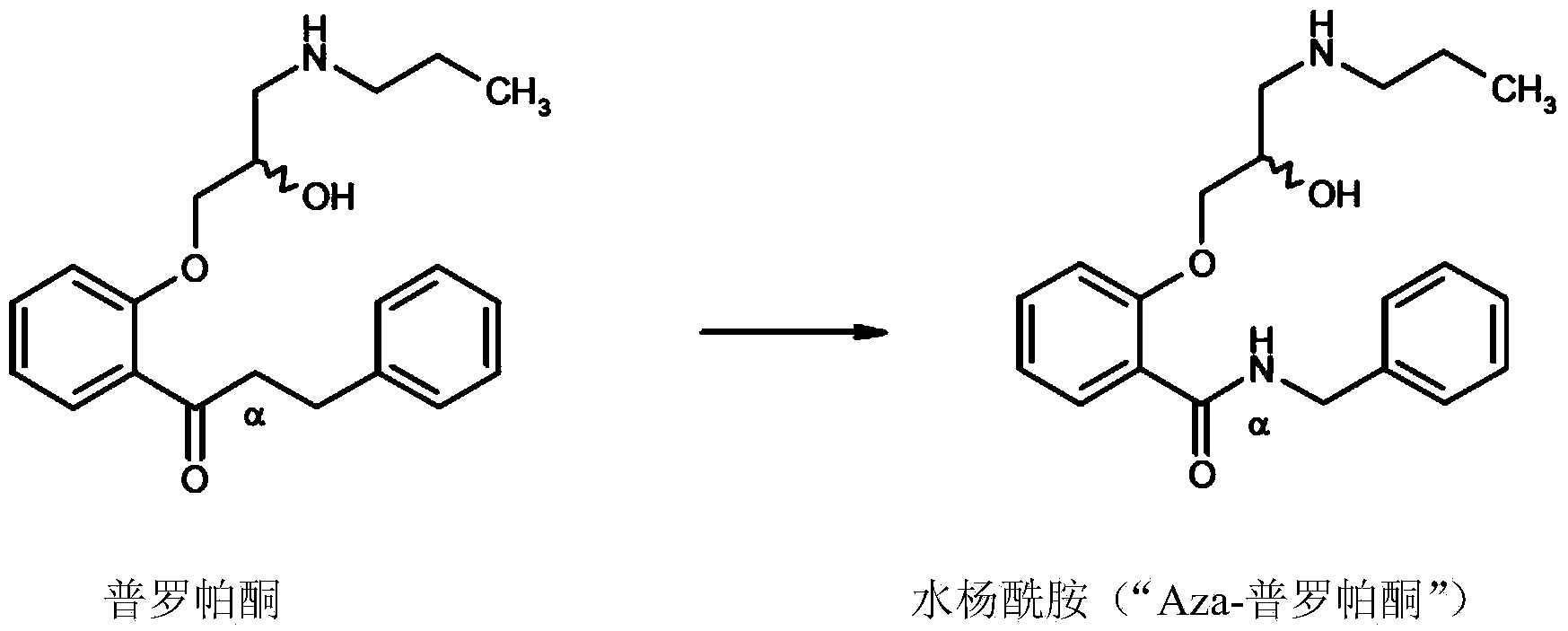
Amidophenoxypropanolamines
An alkoxy and alkyleneoxy technology, which can be used in drug combinations, anti-infectives, active ingredients of heterocyclic compounds, etc., can solve problems such as harmful antimalarial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0519] 2-{3-[(adamantan-1-ylmethyl)-amino]-2-hydroxy-propoxy}-N-p-tolyl-benzamide (compound of formula I-40)
[0520] Compounds were prepared according to Reaction Scheme 1 above.
[0521] Step a1) 2-Hydroxy-N-p-tolyl-benzamide
[0522] A drop of boron trifluoride diethyl ether was added to a solution of phenyl 2-hydroxybenzoate (1 g, 46.7 mmol) and 4-toluidine (0.5 g, 46.7 mmol) in toluene (5 mL). The resulting reaction mixture was left overnight at room temperature. A crystalline precipitate formed and the crystals were collected by filtration, washed 3 times with cold MTBE and dried in vacuo to afford 2-hydroxy-N-p-tolyl-benzamide in the form of colorless crystals (theoretically 0.81 g, 76%). Chemical characterization data correspond to data from known compounds (CAS 7164-80-9).
[0523] Step b1) 2-oxiranylmethoxy-N-p-tolyl-benzamide
[0524] Freshly powdered KOH (0.7 g, 13.2 mmol) was added to a solution of 2-hydroxy-N-p-tolyl-benzamide (3 g, 13.2 mmol) in MeOH (20...
example 2
[0528] 2-{2-Hydroxy-3-[4-(3-trifluoromethyl-3H-diaziridin-3-yl)-benzylamino]propoxy}-N-(3-methyl-butyl Base)-5-propyn-2-yloxy-benzamide (compound of formula I-44)
[0529] This compound is prepared according to the following reaction scheme 4:
[0530] Reaction scheme 4
[0531]
[0532] Previous step a1) 2-Hydroxy-5-propyn-2-yloxy-benzoic acid methyl ester
[0533] Propargyl bromide (30.7 mL, 285 mmol) was added to 2,5-dihydroxy-benzoic acid methyl ester (40 g, 237.9 mmol) and K 2 CO 3 (40 g, 285 mmol) was suspended in acetone (250 mL) and the reaction mixture was kept at reflux for 20 h. The resulting heterogeneous mixture was filtered and volatile materials were removed under reduced pressure. The remaining oil was neutralized with 2N HCl. The obtained mixture was extracted with EtOAc, the organic phase was washed with saturated NaHCO 3 - the solution was washed once with MgSO 4 dry. The solvent was removed from the dried solution under reduced pressure and th...
example 3
[0543] 2-[3-(Adamantan-2-yl-methyl-amino)-2-hydroxy-propoxy]-N-[4-(3-trifluoromethyl-3H-diaziridine-3- Base)-benzyl]-benzamide (compound of formula I-43)
[0544] This compound was prepared according to Reaction Scheme 2 above.
[0545] Step a2) 2-oxiranylmethoxy-methylbenzoate
[0546] Freshly powdered KOH (0.74 g, 13.1 mmol) was added to a solution of methyl 2-hydroxybenzoate (2 g, 13.1 mmol) in MeOH (15 mL) and the mixture was kept at 60 °C with a rotary evaporator. The solvent was removed from the resulting homogeneous solution under reduced pressure. Racemic epichlorohydrin (10 mL) was added and the resulting mixture was heated to reflux for 5 min. Excess epichlorohydrin was removed under reduced pressure and the resulting oil was diluted with EtOAc (30 mL). The obtained dilutions were washed three times with brine, washed with Na 2 SO 4 Drying, concentration under reduced pressure and purification by chromatography (MTBE / PE 3:2) gave 2-oxiranylmethoxy-benzoic aci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com