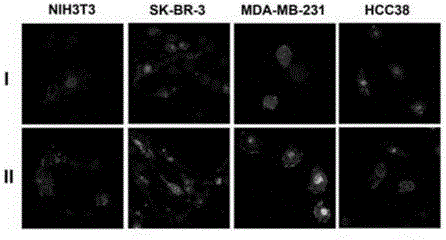
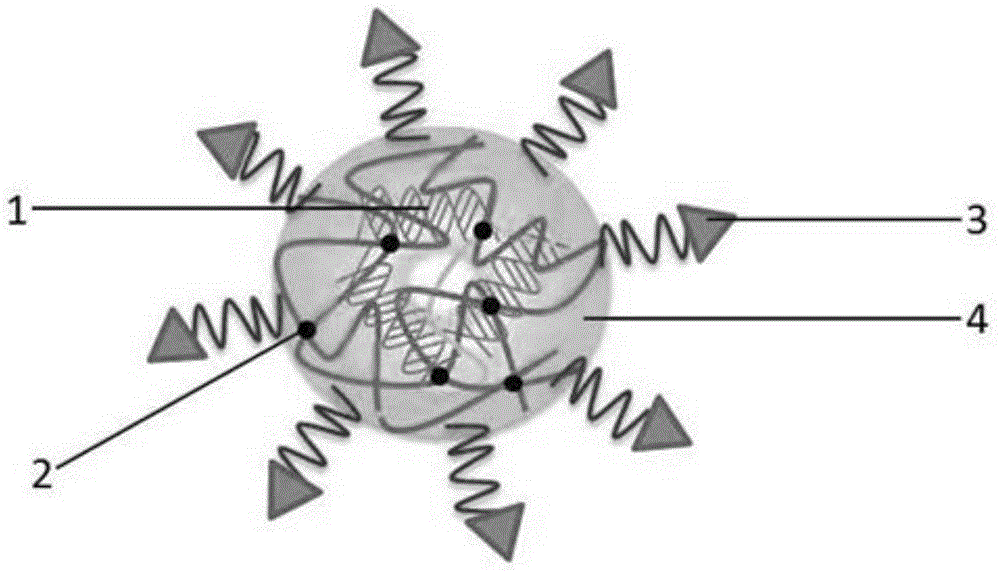
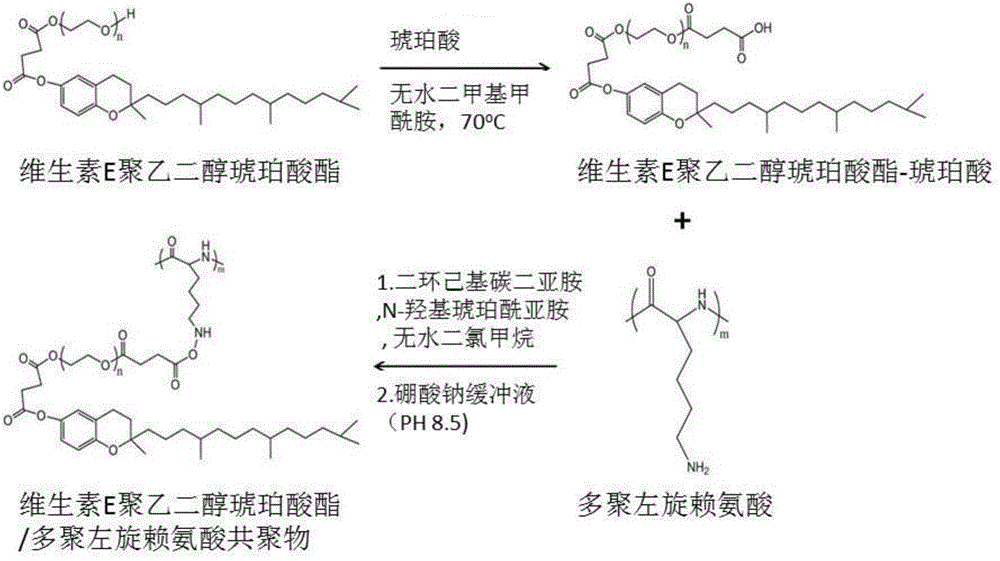
Application of vitamin E polyethylene glycol succinate and derivatives thereof in preparation of hydrogel nanoparticle preparation of prodrug of hydrophilic medicine
A technology of polyethylene glycol succinate and hydrophilic drugs, which is applied in the field of pharmaceutical preparations to achieve strong biocompatibility and reduce non-specific phagocytosis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Vitamin E polyethylene glycol succinate / poly-L-lysine copolymer (TPGS-PLL) is used to prepare the prodrug hydrogel nanoparticle formulation of cisplatin
[0039] Cisplatin is a commonly used anticancer drug approved by the US FDA for the treatment of bladder cancer, cervical cancer, malignant mesothelioma, non-small cell lung cancer, ovarian cancer, head and neck cancer Drugs for squamous cell carcinoma, testicular cancer. Cisplatin has a certain solubility in water, and it is usually dissolved in normal saline (0.9% NaCl) for injection. Cisplatin dissolved in normal saline has an elimination half-life of 1 hour in humans with normal renal function. Usually 2-3 hours after the end of the injection or infusion, 90% of the cisplatin will be irreversibly combined with the protein in the blood and be metabolized. The normal saline preparation of cisplatin has no target, and it will accumulate in various organs of the body in the human body, causing strong side e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com