Macrocyclic diterpenoid compounds separated from euphorbia macrorrhiza C.A.Mey and application
A compound and diterpenoid technology, applied in the field of preparing anti-tumor drugs, can solve the problems of unknown chemical composition of Euphorbia vulgaris
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
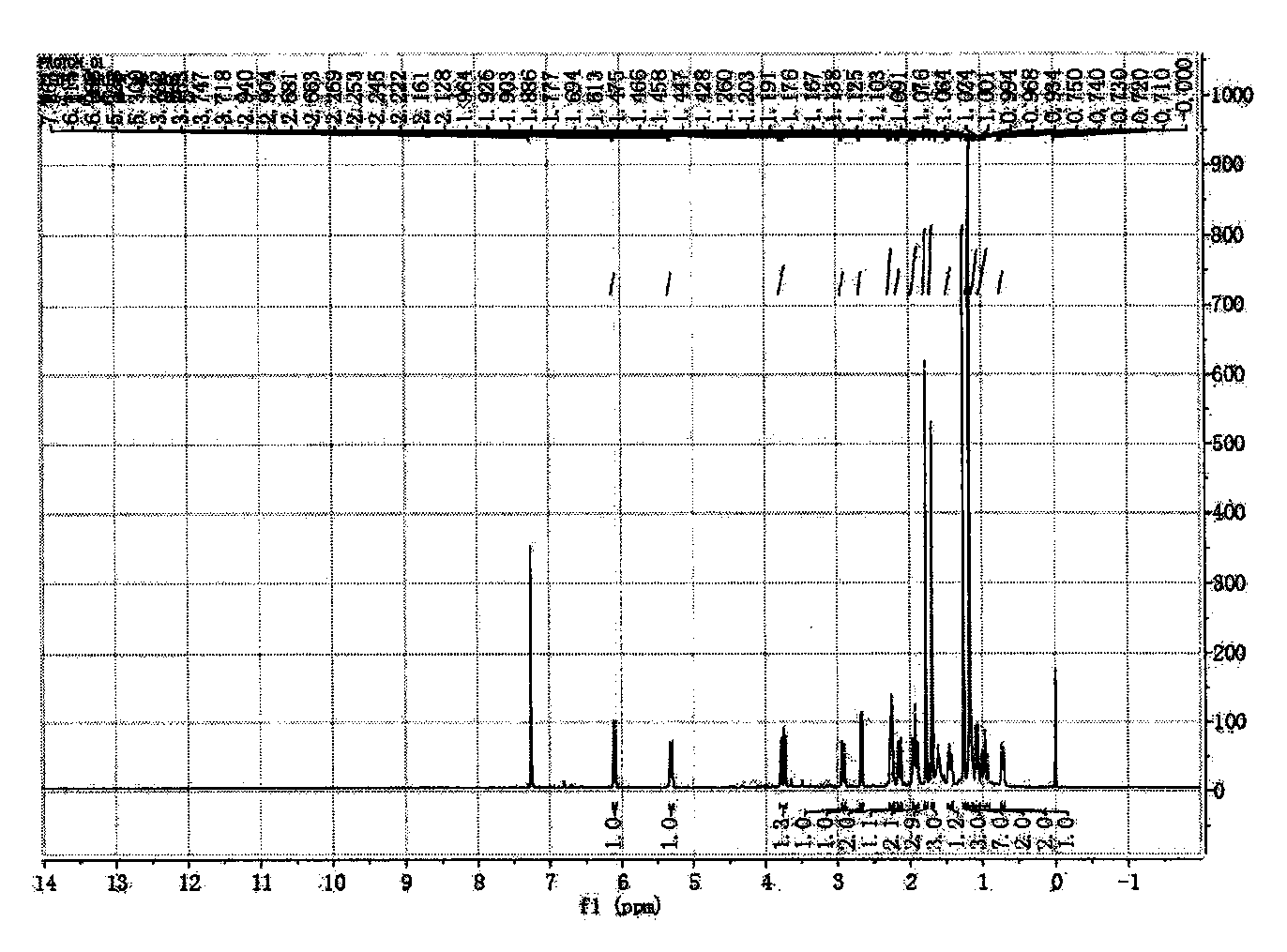
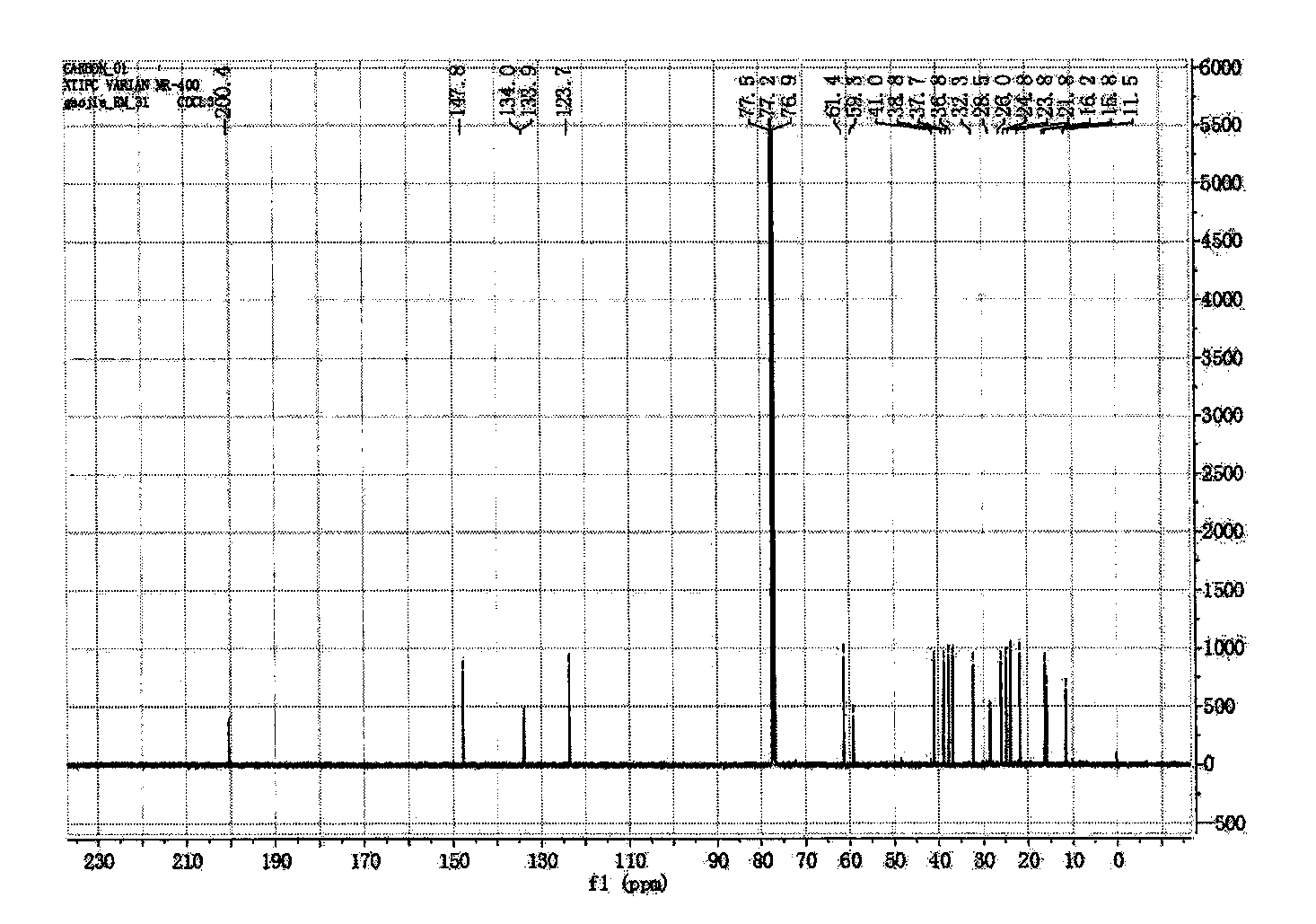
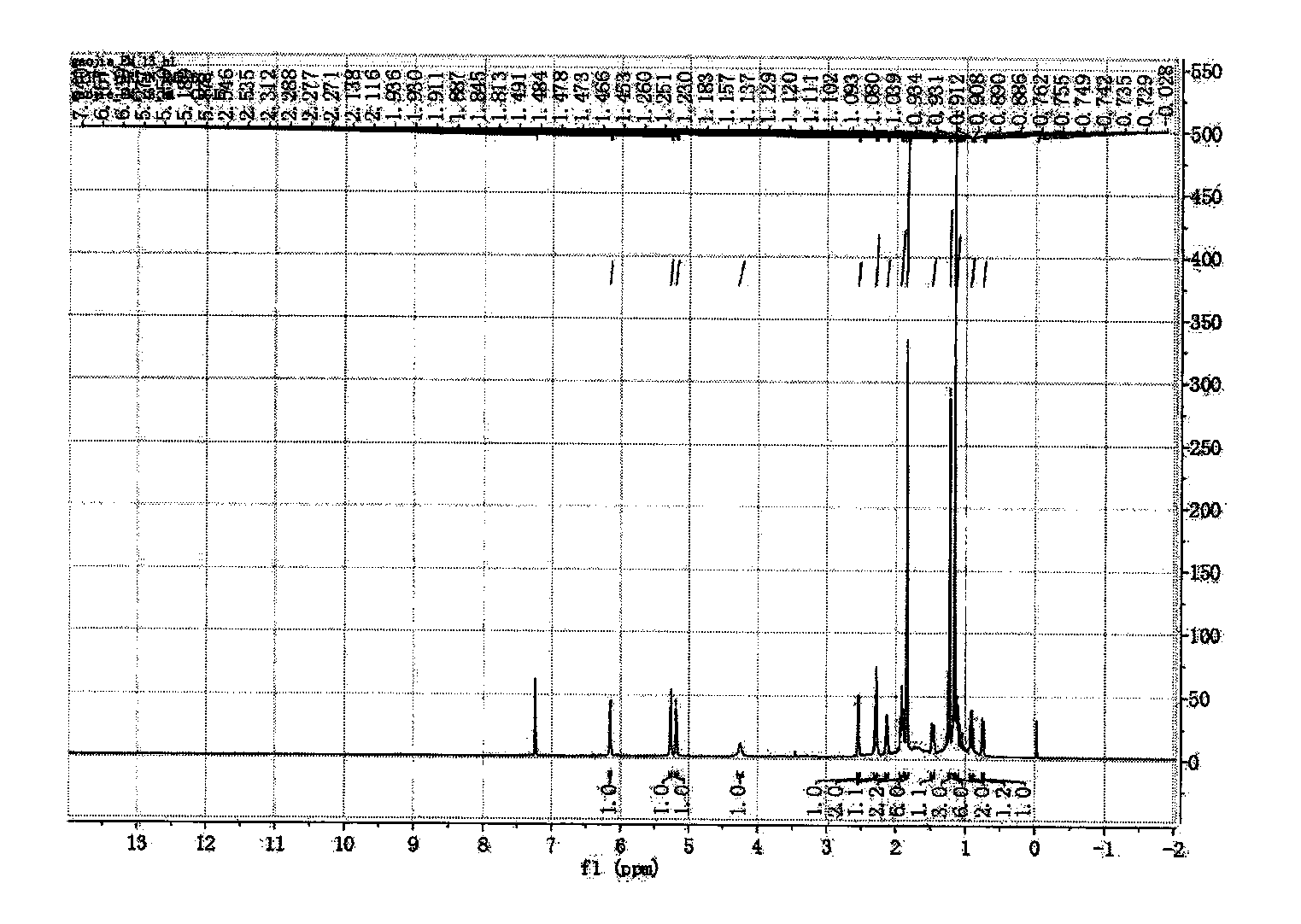
[0046] Preparation of new macrocyclic diterpenoids
[0047] Take 2.4kg of the whole plant of Euphorbia euphorbia, crush it, extract it with 25L acetone percolation method at room temperature, and evaporate the solvent to dryness under reduced pressure to obtain the crude extract of Euphorbia euphorbia; the crude extract is dispersed with cyclohexane, added Acetonitrile is extracted, and the acetonitrile layer is combined, and the acetonitrile is evaporated to dryness under reduced pressure to obtain the acetonitrile extract extract;
[0048] The obtained acetonitrile extract extract was separated by normal phase silica gel column, and gradient elution was carried out with petroleum ether-ethyl acetate=100:1-0:1 as the solvent system, and the fraction was analyzed by silica gel thin layer chromatography (TLC) , combined the same fractions to obtain 11 sections of components (F1-F11), wherein the section F5 components were then separated by a normal phase silica gel column, usin...
Embodiment 2
[0079] The application of the macrocyclic diterpenoids isolated from Euphorbia rhizome in the present invention in the preparation of antitumor drugs is exemplified by human oral epidermoid cancer cell lines and drug-resistant strains.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com