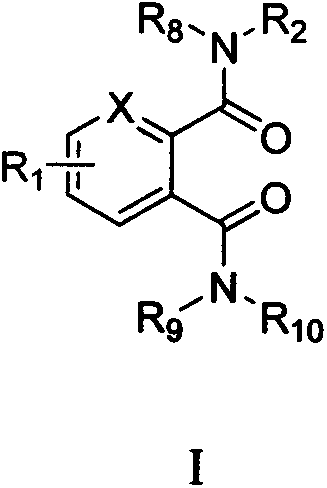
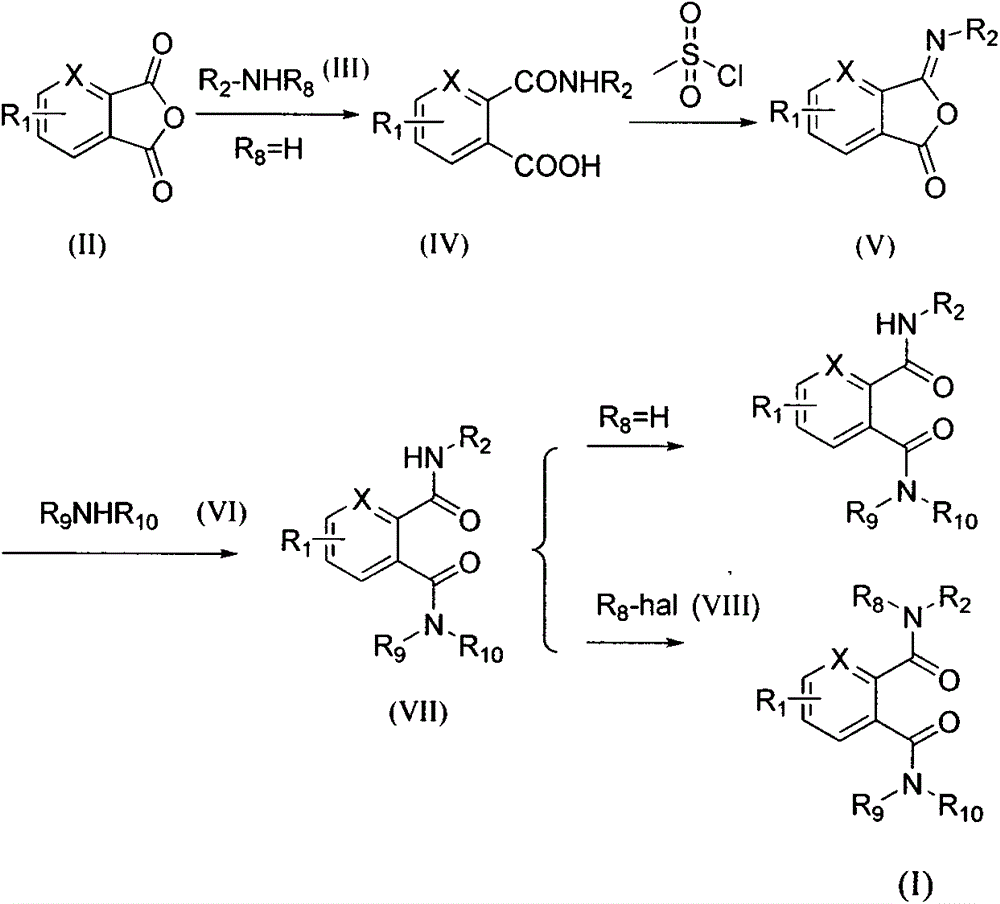
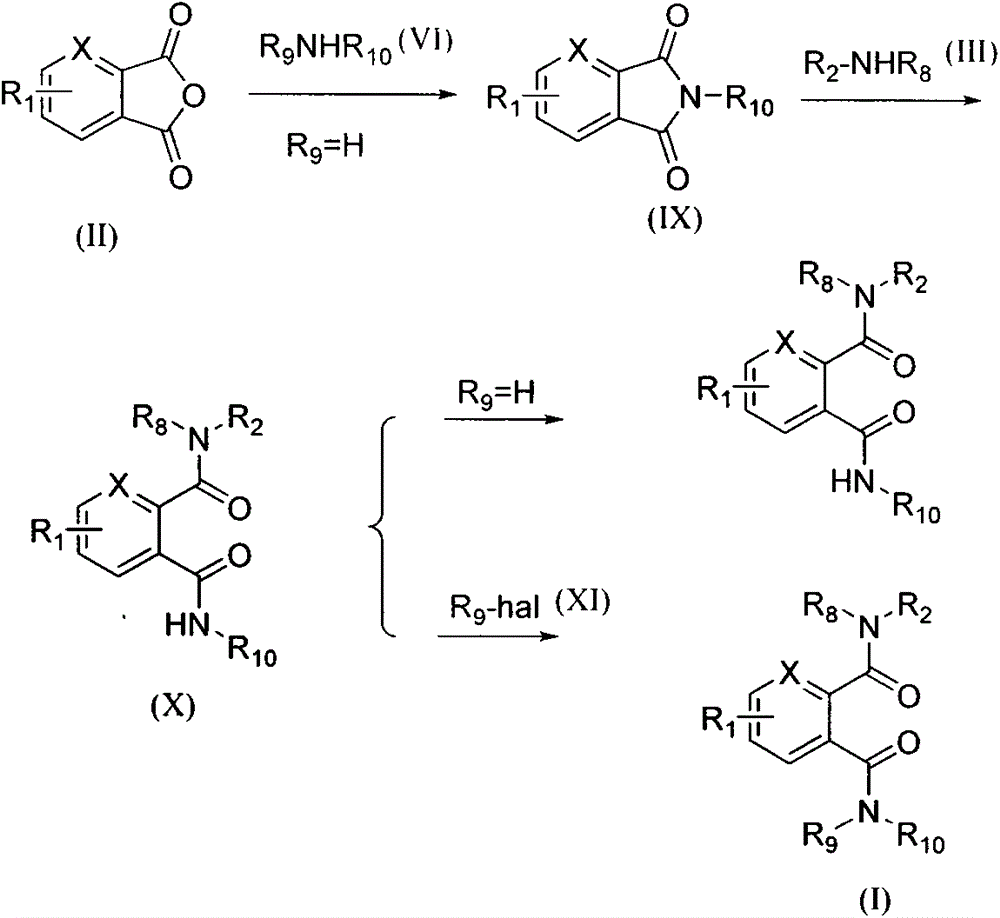
Novel bisamides derivative and preparation method and application thereof
A technology of bisamides and derivatives, which is applied in the field of preparation of new compounds, and can solve the problems that the application of bisamide derivatives with anti-tumor activity has not yet been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] N 1 -(5-(2,2-dichloroacetyl)-2-methylphenyl)-3-iodo-N 2 -Synthesis of (2-methyl-1-(1-thiomethyl)-2-propyl)phthalamide (derivative 01):
[0055]Step A: Preparation of 2-iodo-6-carboxy-N-(1,1-dimethyl-2-methylthioethyl)benzamide
[0056] 3-Iodophthalic anhydride (2.74g), 1-methylthio-2-methyl-2-aminopropane (1.19g) and triethylamine (1.00g) were mixed and dissolved in an appropriate amount of dichloromethane. The reaction mixture was stirred and reacted for 12 h, and worked up to obtain 2-iodo-6-carboxy-N-(1,1-dimethyl-2-methylthioethyl)benzamide as a yellow solid. Yield 75%, m.p. 127-128°C.
[0057] Step B: Preparation of N-acetyl-2-methylaniline.
[0058] 2-Methylaniline (5.1g), triethylamine (10.1g) and acetyl chloride (3.9g) were dissolved in an appropriate amount of dichloromethane, and stirred at room temperature for 2h. Workup gave N-acetyl-2-methylaniline. Yield 95%, m.p.108-109°C.
[0059] Step C: Preparation of N-(5-(2,2-dichloroacetyl)-2-methylphenyl)acet...
Embodiment 2
[0066] N 1 -(5-(2,2-dichloroacetyl)-2-methylphenyl)-3-iodo-N 2 -Synthesis of (2-methyl-1-(1-methylsulfonyl)-2-propyl)phthalamide (derivative 02):
[0067] Will N 1 -(5-(2,2-dichloroacetyl)-2-methylphenyl)-3-iodo-N 2 -(2-methyl-1-(1-thiomethyl)-2-propyl)phthalamide (0.30g), m-chloroperoxybenzoic acid (0.17g) were dissolved in tetrahydrofuran, stirred at room temperature , post-treatment yielded a white end product N 1 -(5-(2,2-dichloroacetyl)-2-methylphenyl)-3-iodo-N 2 -(2-Methyl-1-(1-methylsulfonyl)-2-propyl)phthalamide.
Embodiment 3
[0069] N 1 -(5-(2,2-dichloroacetyl)-2-methylphenyl)-3-iodo-N 2 Synthesis of -(2-methyl-1-(1-(N-aminocarbonylthiocarbonyl)methylthio)-2-propyl)phthalamide (derivative 03):
[0070] In an ice bath, N 1 -(5-(2,2-dichloroacetyl)-2-methylphenyl)-3-iodo-N 2 -(2-Methyl-1-(1-thiomethyl)-2-propyl)phthalamide (0.30g), iodobenzene acetate (0.134g), cyanamide (0.1g, 1.5mmol) Dissolve in tetrahydrofuran, heat up to room temperature and stir, and post-process to obtain a white solid N 1 -(5-(2,2-dichloroacetyl)-2-methylphenyl)-3-iodo-N 2 -(2-Methyl-1-(1-(N-aminocarbonylthiocarbonyl)methylthio)-2-propyl)phthalamide.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com