Preparation method of impurity compound in ramelteon and prepared standard substance
A technology of ramelteon and compound, applied in the field of pharmacy, can solve the problem that there is no formula (I) and/or formula (II) compound standard preparation and sales, no formula (I) and/or formula (II) ) compounds, etc., to achieve the effects of easy availability of raw materials, low cost and suitable yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
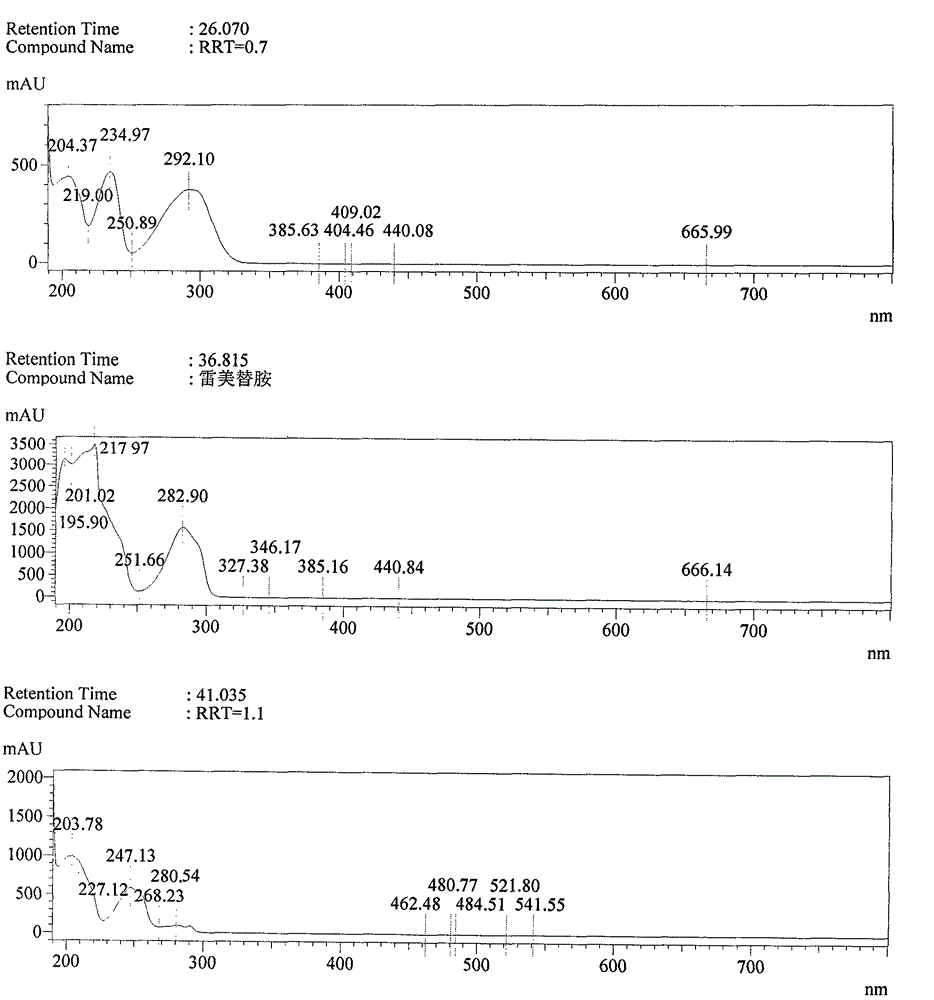
[0080] Step 1, preparation of ramelteon damage sample
[0081] Take 20 grams of ramelteon raw material (manufactured by Zhuhai United Laboratories Co., Ltd., batch number 140218), and place it at 160° C. for 2 hours to generate the impurities.
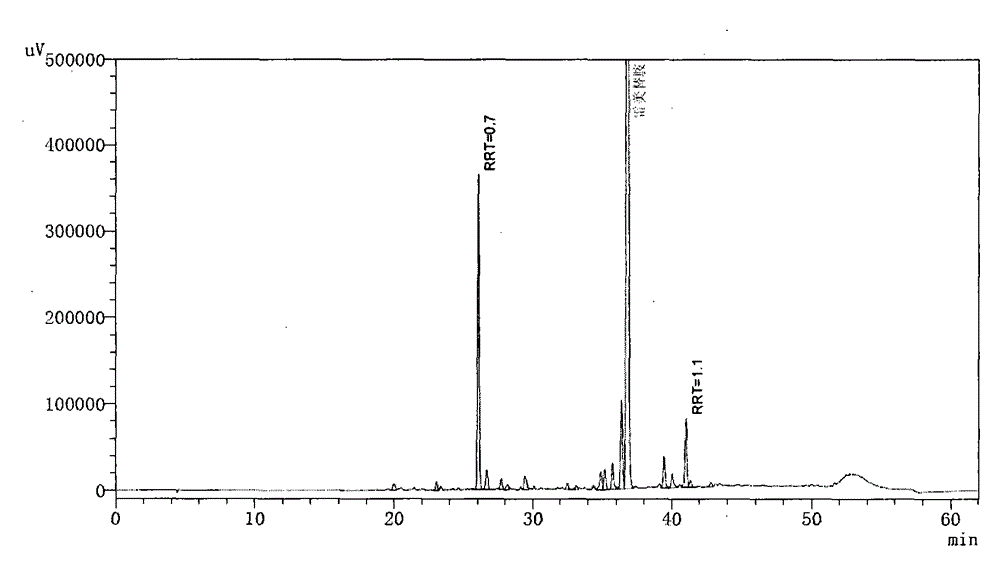
[0082] Step 2, HPLC Determination of Ramelteon Destruction Sample
[0083] High performance liquid chromatography: Shimadzu 20A HPLC (equipped with DAD detector);
[0084] Chromatographic column: Hypersil GOLD 5μ250×4.6mm;
[0085] Mobile phase A: 0.1% formic acid solution; Mobile phase B: acetonitrile;
[0086] Gradient: 0min (A: 90%, B: 10%), 5min (A: 90%, B: 10%), 45min (A: 40%, B: 60%), 50min (A: 10%, B: 90%), 51min (A: 90%, B: 10%), 60min (A: 90%, B: 10%);
[0087] Flow rate: 0.8ml / min;
[0088] Injection volume: 20μL;
[0089] Detection wavelength: 288nm.
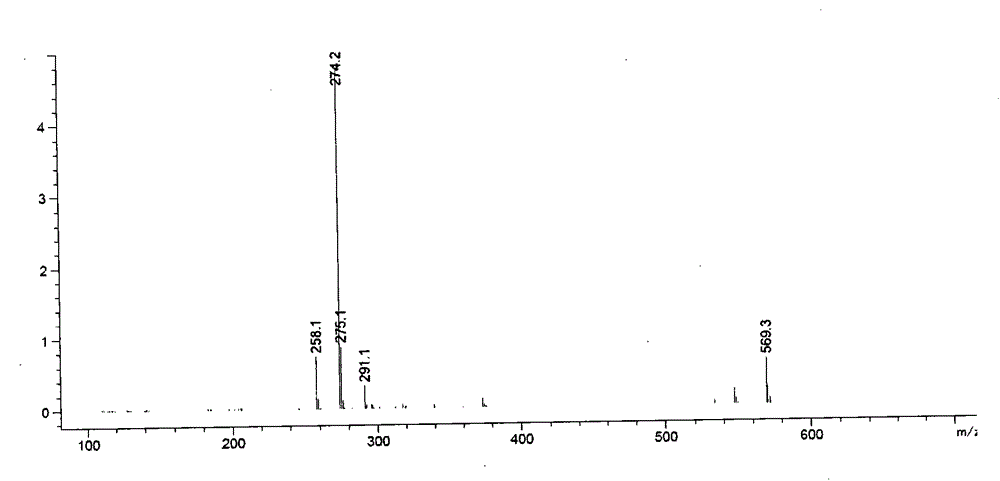
[0090] Get above-mentioned destruction sample appropriate amount, be mixed with the solution that contains ramelteon about 2mg / ml with 50% acetonitrile-water, sample in...
Embodiment 2
[0116] Step 1, preparation of ramelteon damage sample
[0117] Take 10 grams of ramelteon raw material (manufactured by Zhuhai United Laboratories Co., Ltd., batch number 140218), and place it at 180° C. for 3 hours to generate the impurities.
[0118] Step 2, HPLC Determination of Ramelteon Destruction Sample
[0119] High performance liquid chromatography: Shimadzu 20A HPLC (equipped with DAD detector);
[0120] Chromatographic column: Hypersil GOLD 5μ250×4.6mm;
[0121] Mobile phase A: 0.1% formic acid solution; Mobile phase B: acetonitrile;
[0122] Gradient: 0min (A: 90%, B: 10%), 5min (A: 90%, B: 10%), 45min (A: 40%, B: 60%), 50min (A: 10%, B: 90%), 51min (A: 90%, B: 10%), 60min (A: 90%, B: 10%);
[0123] Flow rate: 0.8ml / min;
[0124] Injection volume: 20μL;
[0125] Detection wavelength: 288nm.
[0126] Get above-mentioned destruction sample appropriate amount, be mixed with the solution that contains ramelteon about 2mg / ml with 50% acetonitrile-water, sample in...
Embodiment 3
[0145] Step 1, preparation of ramelteon damage sample
[0146] Take 10 grams of ramelteon raw material (manufactured by Zhuhai United Laboratories Co., Ltd., batch number 140218), and place it at 140° C. for 1.5 hours to generate the impurities.
[0147] Step 2, HPLC Determination of Ramelteon Destruction Sample
[0148] High performance liquid chromatography: Shimadzu 20A HPLC (equipped with DAD detector);
[0149] Chromatographic column: Hypersil GOLD 5μ250×4.6mm;
[0150] Mobile phase A: 0.1% formic acid solution; Mobile phase B: acetonitrile;
[0151] Gradient: 0min (A: 90%, B: 10%), 5min (A: 90%, B: 10%), 45min (A: 40%, B: 60%), 50min (A: 10%, B: 90%), 51min (A: 90%, B: 10%), 60min (A: 90%, B: 10%);
[0152] Flow rate: 0.8ml / min;
[0153] Injection volume: 20μL;
[0154] Detection wavelength: 288nm.
[0155]Get above-mentioned destruction sample appropriate amount, be mixed with the solution that contains ramelteon about 2mg / ml with 50% acetonitrile-water, sample i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com