Metallographic structure etching solution of high-nitrogen stainless steel and metallographic etching method
A high-nitrogen stainless steel and corrosion solution technology, applied in the preparation of test samples, etc., can solve the problems of insufficient metallographic structure boundary, corrosion of high-nitrogen steel metallographic structure, poor structure display effect, etc., and achieve corrosion process Controllable, clear grain boundary, good display effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Use analytical reagents hydrochloric acid, acetic acid, nitric acid and glycerin as raw materials, mix and prepare a solution according to the volume ratio of 35:25:30:10, and set aside.
[0034] (2) Polish the inlaid samples with 200#, 400#, 600#, 800#, 1000# sandpaper, and then polish them with silk cloth sprayed with 2.5μm diamond polishing agent;
[0035] (3) Immerse the polished sample in the corrosion solution and keep it for 120 seconds until the surface of the sample turns silver gray;
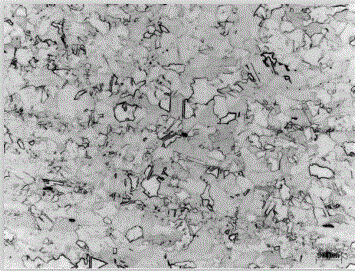
[0036] (4) Rinse the surface of the sample with deionized water immediately after the corrosion is completed, then rinse the surface of the sample with absolute ethanol, and finally dry it with compressed air, and then observe it under a microscope. The metallographic structure of the sample is as follows: figure 1 shown.
Embodiment 2
[0038] (1) Use analytical reagents hydrochloric acid, acetic acid, nitric acid and glycerin as raw materials, mix and prepare a solution according to the volume ratio of 38:27:30:5, and set aside.
[0039] (2) Polish the inlaid samples with 200#, 400#, 600#, 800#, 1000# sandpaper, and then polish them with silk cloth sprayed with 2.5μm diamond polishing agent;
[0040] (3) Immerse the polished sample in the corrosion solution and keep it for 60 seconds until the surface of the sample turns silver gray;
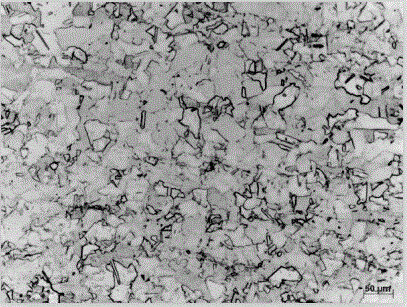
[0041] (4) Rinse the surface of the sample with deionized water immediately after the corrosion is completed, then rinse the surface of the sample with absolute ethanol, and finally dry it with compressed air, and then observe it under a microscope. The metallographic structure of the sample is as follows: figure 2 shown.
Embodiment 3
[0043] (1) Use analytical reagents hydrochloric acid, acetic acid, nitric acid and glycerin as raw materials, mix and prepare a solution according to the volume ratio of 40:30:25:5, and set aside.
[0044] (2) Polish the inlaid samples with 200#, 400#, 600#, 800#, 1000# sandpaper, and then polish them with silk cloth sprayed with 2.5μm diamond polishing agent;
[0045] (3) Use absorbent cotton dipped in corrosive solution to repeatedly wipe the surface of the polished sample until the surface turns silver gray;
[0046] (4) Rinse the surface of the sample with deionized water immediately after the corrosion is completed, then rinse the surface of the sample with absolute ethanol, and finally dry it with compressed air, and then observe it under a microscope. The metallographic structure of the sample is as follows: image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com