Method for green catalytic synthesis of nitrobenzaldehyde
A technology of nitrobenzaldehyde and nitrotoluene, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry and other directions, can solve problems such as hydrogen bromide pollution, and achieves reduction of catalytic cost, low reaction conditions, Inexpensive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
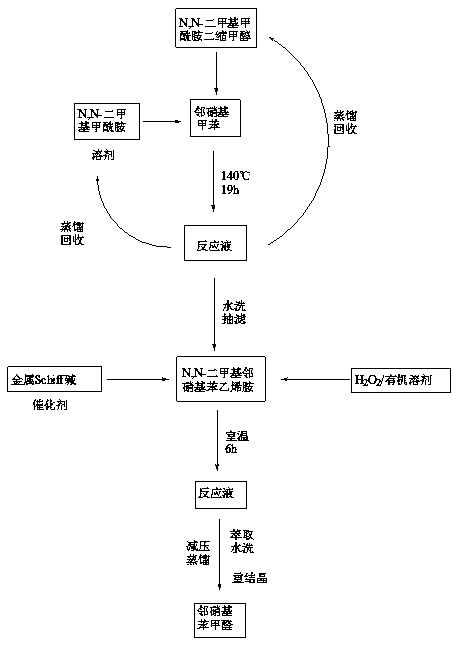
[0023] 1) Add o-nitrotoluene (0.1mol, 13.7g), N,N-dimethylformamide diacetal (0.2mol, 26ml) and N,N-dimethylformamide (75ml) into the reactor , heated to reflux, and reacted for 19h.
[0024] 2) After the reaction is completed, cool down to room temperature to obtain a deep red N,N-dimethyl-o-nitrostyrylamine solution, and recover N,N-dimethylformamide diacetal by atmospheric distillation, and then recover N by distillation under reduced pressure. , N-dimethylformamide, the residual temperature was lowered to room temperature, slowly added 150ml of water, stirred and filtered with suction to obtain N,N-dimethyl-o-nitrostyrylamine, which was directly used in the next step.
[0025] 3) Dissolve N,N'-disalicylaldehyde ethylenediamine manganese (0.005mol) and N,N-dimethyl-o-nitrostyrylamine prepared in the previous step in 250ml of acetonitrile, cool in ice water, slowly drop Add 60ml of 30%wt hydrogen peroxide (0.6mol), drop it within 30min, remove the ice bath, and react at roo...
Embodiment 2
[0028] The process was the same as in Example 1, except that the o-nitrotoluene in the first step was changed to p-nitrotoluene, the toluene in the fourth step was changed to n-hexane, and other factors remained unchanged to obtain 11.0 g of yellow crystals. Melting point: 105.6-106.8°C, yield 74.2% (calculated as p-nitrobenzaldehyde), purity (HPLC): 98.2%.
Embodiment 3
[0030] The process was the same as in Example 1, except that the o-nitrotoluene in the first step was changed to m-nitrotoluene, and the toluene in the fourth step was changed to cyclohexane, and other factors remained unchanged to obtain 6.28 g of yellow crystals. Melting point: 58-59.2°C, yield 43.1% (calculated as p-nitrobenzaldehyde), purity (HPLC): 96.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com