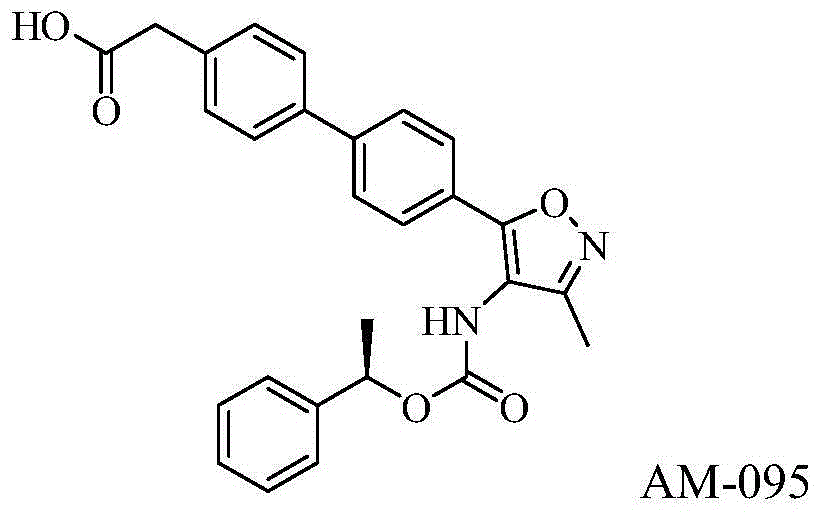
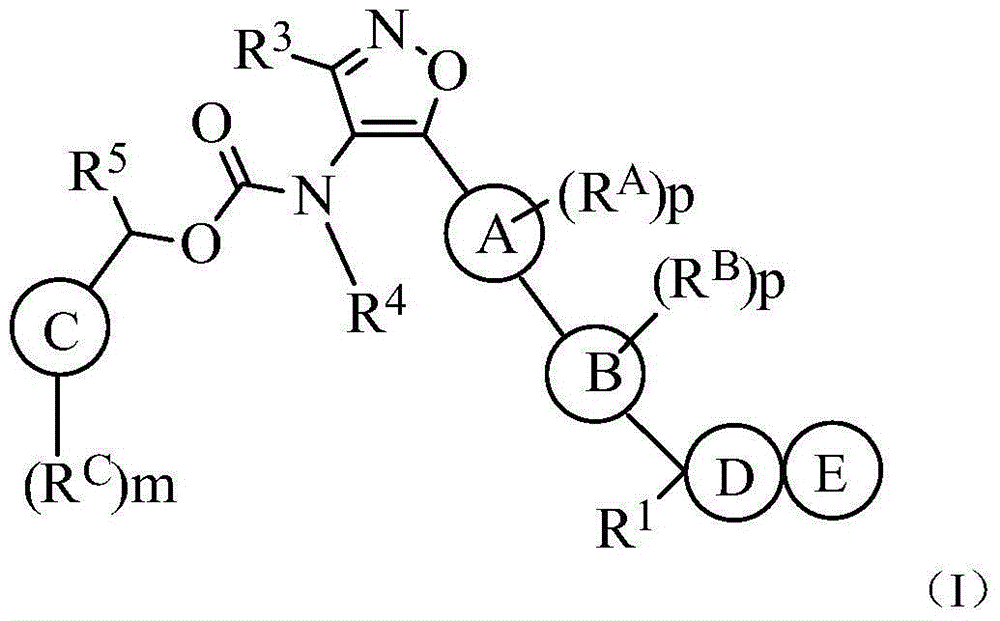
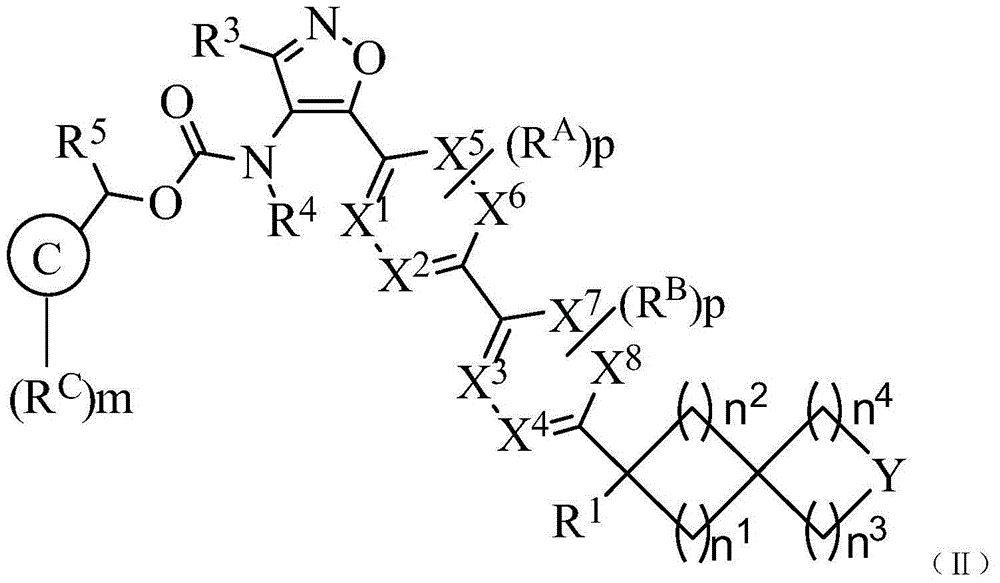
Carboxylic acid derivative as lysophosphatidic acid receptor antagonist
A technology for compounds and stereoisomers, applied in the field of carboxylic acid derivatives as lysophosphatidic acid receptor antagonists, can solve the problems of short half-life and troublesome compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0176] Example 1 2-(4'-(3-methyl-4-(((R)-(1-phenylethoxy)carbonylamino)isoxazol-5-yl)biphenyl)-4-yl ) Preparation of spiro[3.3]heptane-2-carboxylic acid (compound 1)
[0177]
[0178] (1) Preparation of cyclobutyl-1,1-dimethanol
[0179]
[0180] In a 500mL reaction flask, cyclobutyl-1,1-dicarboxylic acid (14.4g, 0.1mol) was dissolved in 300mL THF, and LiALH4 (15.2g, 0.4mol) was added in batches under an ice-water bath. After reacting for 2 hours, quenched with ethyl acetate, added 4N hydrochloric acid to dissolve in clarification, separated liquids, dried and concentrated to obtain 11 g of crude product. The yield was 95%, and the product was directly used in the next reaction.
[0181] (2) Preparation of 1,1-bis(methylsulfonylmethylene)cyclobutane
[0182]
[0183] In a 500mL reaction flask, dissolve cyclobutyl-1,1-dimethanol (11g, 95mmol) in 300mL DCM, add methanesulfonyl chloride (22.9g, 0.2mol) dropwise under an ice-water bath, and react at room temperature ...
Embodiment 2
[0201] Example 2 2-(4'-4-((1-(4-fluorophenyl)ethoxy)carbonylamino)-3-methylisoxazol-5-yl)biphenyl)-4-yl ) Preparation of spiro[3.3]heptane-2-carboxylic acid (compound 2)
[0202]
[0203] (1) 3-methyl-5-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)isoxazole-4 -ylcarbamate-(1-(4-fluorophenyl)ethyl ester)
[0204]
[0205] The operation is the same as in Example 1 (5), and the product is directly used in the next reaction without purification.
[0206] (2) 2-(4'-4-((1-(4-fluorophenyl)ethoxy)carbonylamino)-3-methylisoxazol-5-yl)biphenyl)-4-yl ) ethyl spiro[3.3]heptane-2-carboxylate
[0207]
[0208] Operation is the same as in Example 1 (6), and the productive rate is 54%.
[0209] (3) 2-(4'-4-((1-(4-fluorophenyl)ethoxy)carbonylamino)-3-methylisoxazol-5-yl)biphenyl)-4-yl ) spiro[3.3]heptane-2-carboxylic acid
[0210]
[0211] Operation is the same as Example 1(7), and the productive rate is 2.5%.
[0212] Mass Spectrum (m / e): 555.2 (M+1)
[0213] ...
Embodiment 3
[0214] Example 3 2-(4'-4-((1-(2-fluorophenyl)ethoxy)carbonylamino)-3-methylisoxazol-5-yl)biphenyl)-4-yl ) Preparation of spiro[3.3]heptane-2-carboxylic acid (compound 3)
[0215]
[0216] (1) 2-(4'-4-((1-(2-fluorophenyl)ethoxy)carbonylamino)-3-methylisoxazol-5-yl)biphenyl)-4-yl ) ethyl spiro[3.3]heptane-2-carboxylate
[0217]
[0218] Operation is the same as in Example 1 (6), and the productive rate is 62%.
[0219] (2) 2-(4'-4-((1-(2-fluorophenyl)ethoxy)carbonylamino)-3-methylisoxazol-5-yl)biphenyl)-4-yl ) spiro[3.3]heptane-2-carboxylic acid
[0220]
[0221] Operation is the same as in Example 1 (7), and the productive rate is 25%.
[0222] Mass Spectrum (m / e): 555.2 (M+1)
[0223] 1 H-NMR (400MHz, CDCl 3 ,δppm)δ:1.40-1.50(m,3H),1.78-1.93(m,4H),2.10-2.20(m,2H),2.20-2.30(m,3H),2.50-2.60(d,2H), 2.95-3.05(d,2H),6.00-6.20(m,1H),6.90-7.20(m,3H),7.30(m,1H),7.40-7.50(m,2H),7.52-7.64(m,4H ),7.75-7.83(s,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com