Method for preparing novel animal model of asthma
An animal model and asthma technology, applied in the field of medicine, can solve problems such as poor responsiveness, no visible, weakened bronchospasm, etc., and achieve the effect of good animal responsiveness and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1, the preparation of asthma animal model
[0048] Experimental materials: egg protein (purchased from Sigma-Aldrich Company of the United States), dry powder of aluminum hydroxide (purchased from Sigma-Aldrich Company of the United States), normal saline, Norwegian brown rats of 6-8 weeks (purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. limited company)
[0049] Preparation:
[0050] 1. Mix 25mg of egg protein and 250mg of aluminum hydroxide dry powder and add 1mL of normal saline to make a sensitization solution;
[0051] 2. Take 0.5 mg of egg protein and add 10 mL of normal saline to make 10 mL of 5% egg protein-normal saline solution;
[0052] 3. Take Norway brown rats of 6 to 8 weeks old, and inject the sensitizing solution subcutaneously at two points, 0.2ml per point;
[0053] 4. On the 5th day, repeatedly inject the sensitizing solution subcutaneously in rats at two points, 0.2ml per point;
[0054] 5. On the 37th day...
Embodiment 2
[0057] Embodiment 2, animal model identification of asthma
[0058] Animals were given blood samples before ovalbumin sensitization injection and on the 7th, 14th, 21st, 28th and 35th day after the injection to detect specific IgE. The results showed that IgE appeared from day 14 and peaked at day 28.
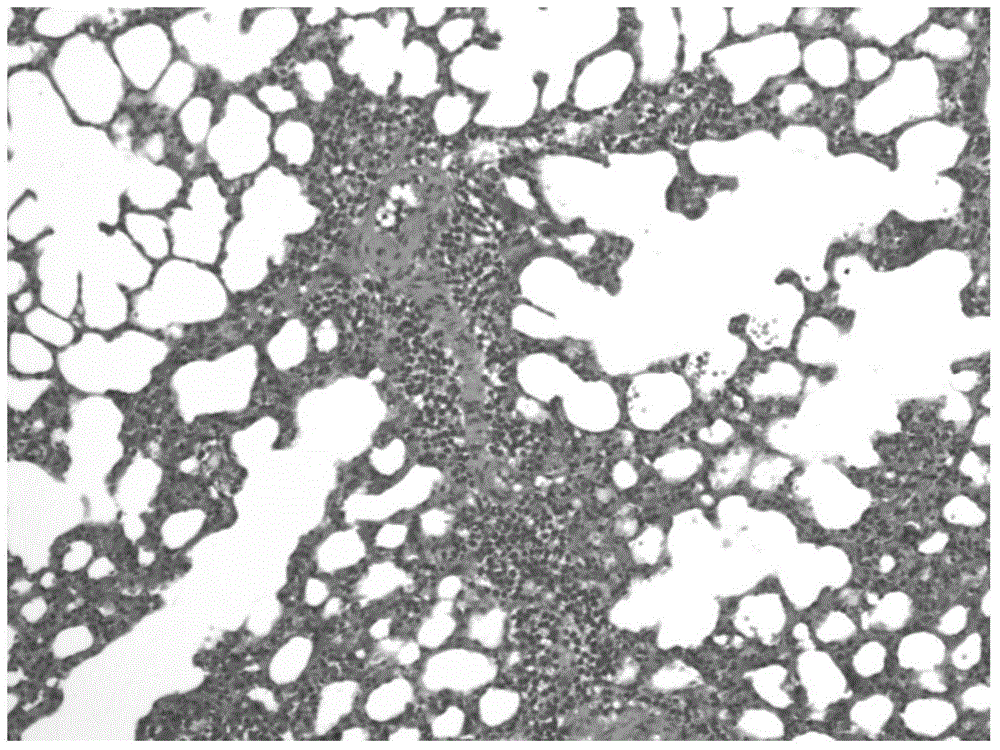
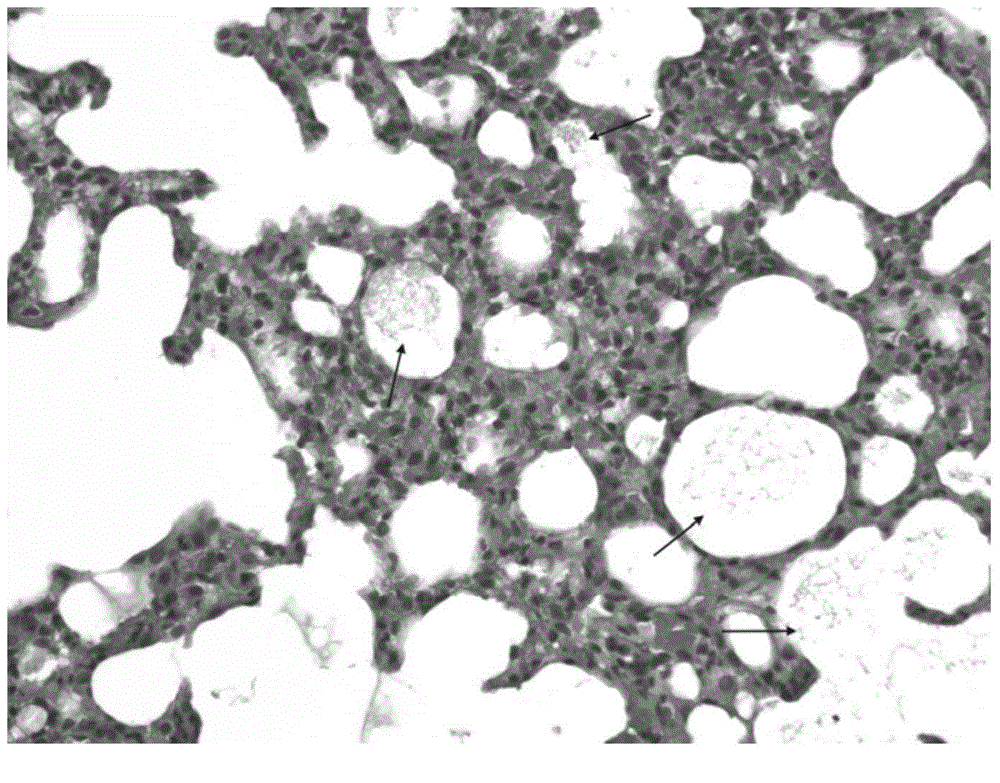
[0059] 24 hours after the asthma attack was provoked, the lungs of the animals were taken out and observed under a HE staining optical microscope. Eosinophilic leukocyte infiltration, increased mucus secretion and focal hemorrhage were seen in the bronchi and alveolar cavities, which were consistent with the inflammatory changes of allergic bronchial asthma attack. see results image 3 , 4 .
Embodiment 3
[0060] Embodiment 3, the method for screening the medicine for the treatment of asthma
[0061] Experimental materials: 6 to 8-week-old inbred Norwegian brown rat (purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.), dexamethasone sodium phosphate injection (purchased from: Tianjin Jinyao Group Hubei Tianyao Pharmaceutical Co., Ltd. company).
[0062] The inbred Norwegian brown rats of 6 to 8 weeks were divided into three groups: normal saline control group, normal control group, and test drug group. According to the method of Example 1, the normal saline control group and the test drug group were sensitized with egg protein injection. , asthma was stimulated by atomization of ovalbumin solution; the normal control group was injected with normal saline, and stimulated by atomized inhalation of normal saline, and then all were recorded by non-invasive whole body plethysmography detector for 16-20 hours. The results of the drug group to be tested are s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com