A kind of synthetic method of mercapto functionalized polyaryl carboxylic acid compound
A technology of aryl carboxylic acid and aryl fluoride, which is applied in the field of synthesis of mercapto-functionalized aryl carboxylic acid compounds, can solve the problems of long reaction cycle, insufficient universality, cumbersome operation, etc., shorten the reaction time, improve The effect of universality and simplified reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
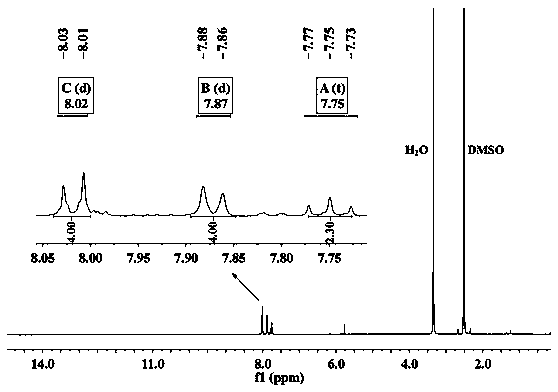
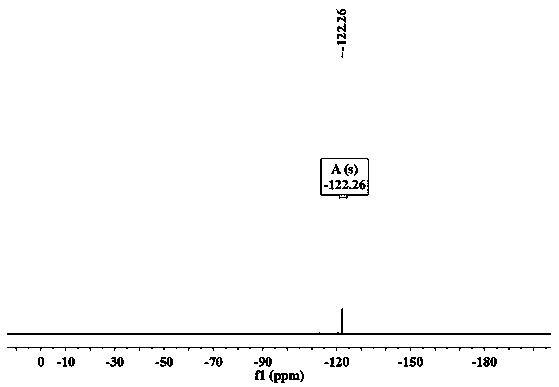
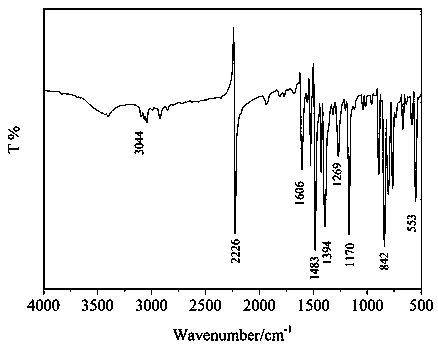
[0048] Example 12', 5'-dimercapto-p-terphenyl-4', the synthesis identification of 4'-dicarboxylic acid
[0049] (1) Synthesis and identification of 2',5'-difluoro-terphenyl-4',4'-dinitrile
[0050] The first is the traditional method (method 1), 1,4-difluoro-2,5-dibromobenzene (1089mg, 4mmol), 4-cyanophenylboronic acid (1293mg, 8.8mmol), potassium carbonate (2210mg, 16mmol) and a catalytic amount of palladium compound 50mL in a single-necked flask, under the protection of nitrogen, add 40mL of deoxygenated toluene aqueous solution, reflux at 90°C for 2 days, separate and purify by silica gel chromatography, and obtain 443mg of a white powdery product (Intermediate 1), Yield 35%, ie intermediate 1.
[0051] This example uses the following method (method 2) to synthesize intermediate 1
[0052] In the new method of the present invention, 1,4-difluoro-2,5-dibromobenzene (1088mg, 4mmol), 4-cyanophenylboronic acid (1292mg, 8.8mmol), potassium carbonate (2208mg, 16mmol) and cataly...
Embodiment 22
[0061] Example 22', 5'-dimercapto-p-terphenyl-4', 4'-dicarboxylic acid synthesis identification
[0062] The synthesis method is the same as in Example 1, the only difference is that when synthesizing intermediate 1, the organic solvent in the water / organic solvent mixed system used is polyethylene glycol 400, and the volume ratio of water and polyethylene glycol 400 is defined For factor E.
Embodiment 32
[0063] Example 32', 5'-dimercapto-p-terphenyl-4', the synthesis identification of 4'-dicarboxylic acid
[0064] The synthesis method is the same as in Example 1, the only difference is that when synthesizing intermediate 1, the organic solvent in the water / organic solvent mixed system used is polyethylene glycol 600, and the volume ratio of water and polyethylene glycol 600 is defined For factor F.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com