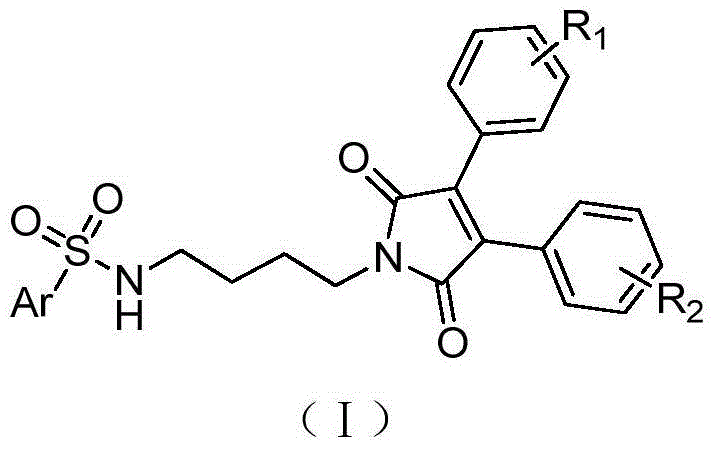
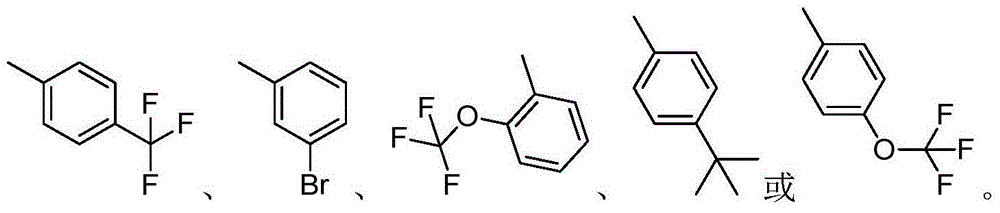
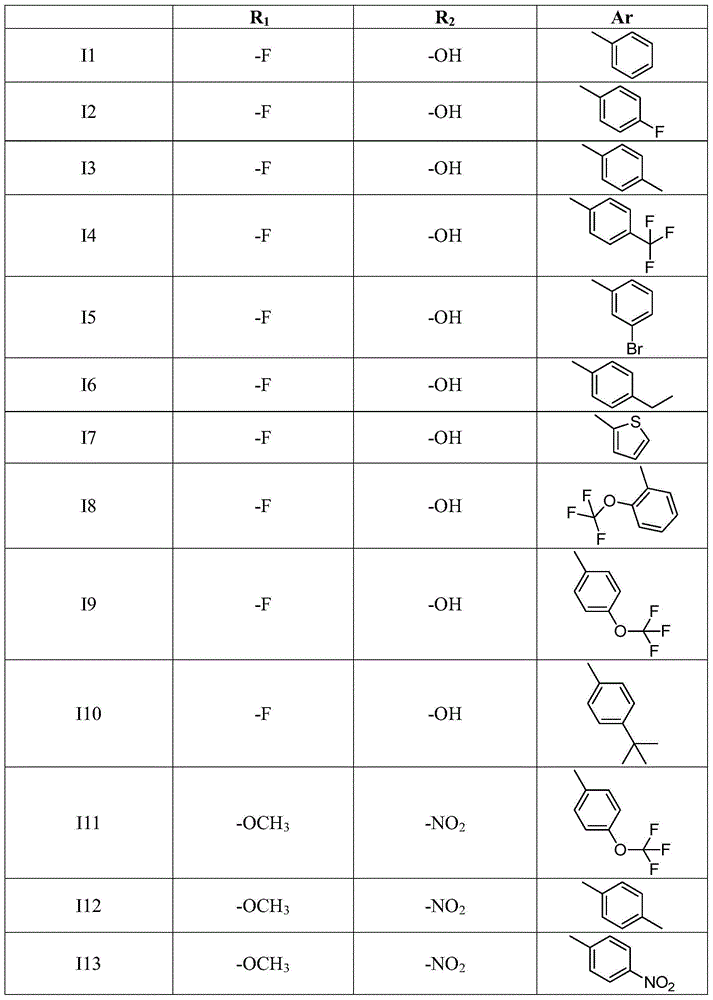
3,4-diarylmaleimide derivative and preparation method as well as application thereof
A technology of maleimide and derivatives, applied in the field of 3,4-diarylmaleimide derivatives and its preparation, can solve problems such as ineffective reversal of atherosclerosis and safety risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: Preparation of α-bromo-p-fluoroacetophenone.
[0067] Dissolve 10g (72.4mmol) of p-fluoroacetophenone in 100mL of acetonitrile, and add 11.57g (72.4mmol) of Br dropwise under stirring 2 acetonitrile solution, stirred overnight (8-10h), after the reaction is complete, remove the solvent by rotary evaporation under reduced pressure, redissolve in ethyl acetate, wash with brine three times, dry over anhydrous sodium sulfate, filter, remove the solvent by rotary evaporation under reduced pressure, and use 50mL Add a few drops of diethyl ether to cyclohexane to make a slurry and filter to obtain 14.2 g of white crystals, with a yield of 90.8%.
[0068] 1 H NMR (300M, CDCl 3 ):δ=4.41(s,2H,-CH 2 -), 7.17(d, J=8.4Hz, 2H, Ar-H), 8.03(dd, J=5.4, 8.7Hz, 2H, Ar-H);
[0069] MS(EI,m / z):[M - ] 215.0.
Embodiment 2
[0070] Example 2: Preparation of α-bromo-p-methoxyacetophenone.
[0071] 12.80g (85.24mmol) of p-methoxyacetophenone was dissolved in 150mL of acetonitrile, and 13.62g (85.24mmol) of Br 2 acetonitrile solution, stirred overnight (8-10h), after the reaction is complete, remove the solvent by rotary evaporation under reduced pressure, redissolve in ethyl acetate, wash with brine three times, dry over anhydrous sodium sulfate, filter, remove the solvent by rotary evaporation under reduced pressure, and use 50mL Add a few drops of diethyl ether to cyclohexane to make a slurry and filter to obtain 14.31 g of white crystals, yield: 73.5%.
[0072] 1 H NMR (500M, CDCl 3 ):δ=3.89(s,3H,-OCH 3 ),4.40(s,2H,-CH 2 -), 6.96(d, J=8.5Hz, 2H, Ar-H), 7.97(d, J=9Hz, 2H, Ar-H);
[0073] MS(EI,m / z):[M - ] 226.9.
Embodiment 3
[0074] Example 3: Preparation of 2-azido-(4-fluorophenyl)ethanone.
[0075]Dissolve 13.09g (60mmol) of α-bromo-p-fluoroacetophenone in 80mL of methanol, cool down to 0°C, and add 4.71g (72mmol) of NaN 3 Aqueous solution, reaction 4h. After the reaction was complete, 200 mL of water was added under ice-bath conditions, and a white solid precipitated out. The product was obtained by direct filtration, and dried to obtain 10.6 g of product, yield: 98%.
[0076] 1 H NMR (300M, CDCl 3 ):δ=4.52(s,2H,-CH 2 -), 7.18(d, J=8.4Hz, 2H, Ar-H), 7.95(dd, J=5.4, 8.7Hz, 2H, Ar-H);
[0077] MS(EI,m / z):[M + ] 179.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com