Non-imidazole ionic liquid for proton exchange membrane fuel cell electrolyte
A proton exchange membrane and fuel cell technology, applied in solid electrolyte fuel cells, fuel cells, circuits, etc., can solve problems such as relatively difficult, less applied research, and low cation stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
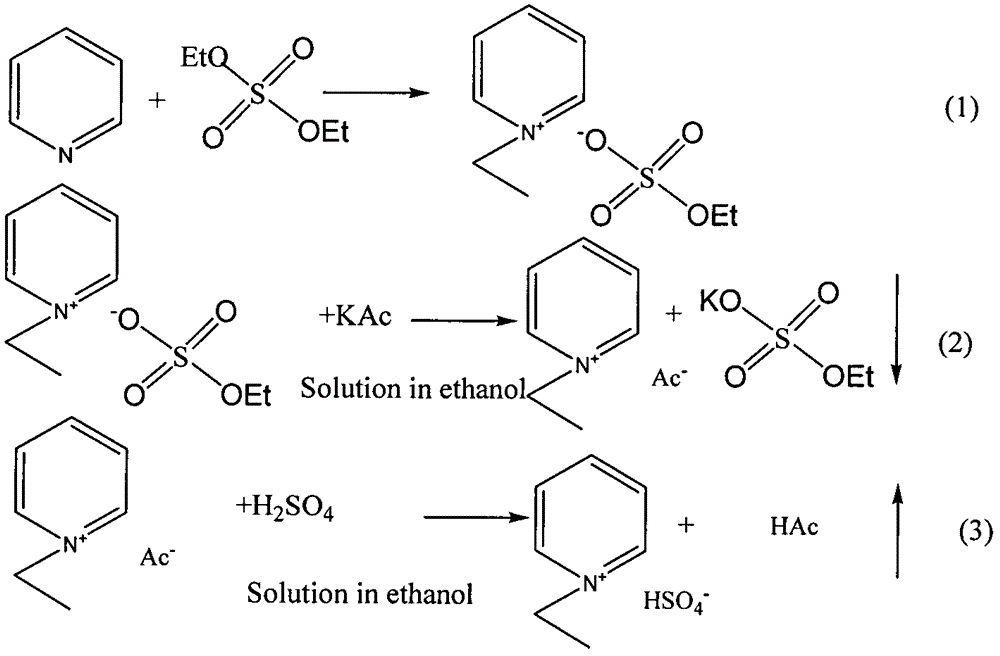
[0016] Example 1: Ethylpyridine hydrogen sulfate ([Epdy]HSO 4 )synthesis
[0017] (1) Add 0.1 mol of pyridine to a single-neck flask, and then add 0.1 mol of diethyl sulfate. After the addition, heat and stir in a water bath at 50° C. for about 12 hours to obtain an aqueous solution of a colorless transparent ionic liquid.
[0018] (2) The above liquid and 0.15 mol KAC were separately dissolved in ethanol, and then the two solutions were mixed to remove the resulting white precipitate, and the filtrate was collected to obtain a colorless liquid.
[0019] (3) Add 0.15 mole of H to the solution 2 SO 4 , Stir evenly to obtain a colorless transparent liquid. The liquid is evaporated in a rotary evaporator to remove most of the water ethanol and the produced acetic acid to obtain a light yellow viscous liquid, which can be dried in vacuum to obtain a more viscous light yellow liquid, which is the product.
Embodiment 2
[0020] Example 2: Butylpyridine hydrogen sulfate ([Bpdy]HSO 4 )synthesis
[0021] (1) Add 0.1 mol of pyridine to a single-necked flask, and then add 0.1 mol of dibutyl sulfate. After the addition, heat and stir in a water bath at 50° C. for about 12 hours to obtain an aqueous solution of a colorless transparent ionic liquid.
[0022] (2) The above liquid and 0.15 mol KAC were separately dissolved in ethanol, and then the two solutions were mixed to remove the resulting white precipitate, and the filtrate was collected to obtain a colorless liquid.
[0023] (3) Add 0.15 mole of H to the solution 2 SO 4 , Stir evenly to obtain a colorless transparent liquid. The liquid is evaporated in a rotary evaporator to remove most of the water ethanol and the produced acetic acid to obtain a light yellow viscous liquid, which can be dried in vacuum to obtain a more viscous light yellow liquid, which is the product.
Embodiment 3
[0024] Example 3: Propylpyridine hydrogen sulfate ([Ppdy]H 2 PO 4 )synthesis
[0025] (1) Add 0.1 mol of ethyl pyridine in a single-necked flask, and then add 0.1 mol of dipropyl sulfate. After the addition, heat and stir in a water bath at 50° C. for about 12 hours to obtain a colorless transparent ionic liquid aqueous solution.
[0026] (2) The above liquid and 0.15 mol KAC were separately dissolved in ethanol, and then the two solutions were mixed to remove the resulting white precipitate, and the filtrate was collected to obtain a colorless liquid.
[0027] (3) Add 0.15 mole of H to the solution 2 SO 4 , Stir evenly to obtain a colorless transparent liquid. The liquid is evaporated in a rotary evaporator to remove most of the water ethanol and the produced acetic acid to obtain a light yellow viscous liquid, which can be dried in vacuum to obtain a more viscous light yellow liquid, which is the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com