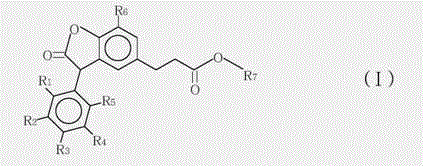
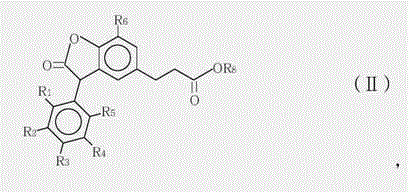
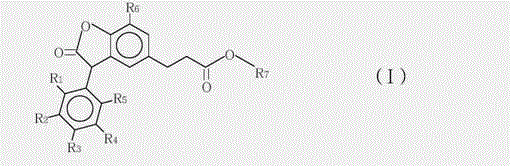
3-arylbenzofuranone compound and composition formed thereby
A compound, furanone technology, applied in chemical materials and its application fields, can solve unsatisfactory problems and achieve good convenience, high molecular weight, and good application compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Preparation of 3-(7-tert-butyl-3-(2,3-dimethylphenyl)-2-oxo-2,3-dihydrobenzofuran-5-yl)-propionic acid methyl ester and 3 - Mixtures of methyl (7-tert-butyl-3-(3,4-dimethylphenyl)-2-oxo-2,3-dihydrobenzofuran-5-yl)propanoate.
[0030] In a four-neck round bottom 1000mL flask equipped with a mechanical stirrer, thermal controller and a reflux condenser, add 300g of 1,2-dichloroethane and 118g of methyl 3-(3-tert-butyl-4- Hydroxyphenyl) propionate, stir well to dissolve methyl 3-(3-tert-butyl-4-hydroxyphenyl)propionate in 1,2-dichloroethane. Then 89 g of glyoxylic acid (50% glyoxylic acid in water) and 0.9 g of p-toluenesulfonic acid monohydrate were added. At 85°C, the mixture was heated and refluxed for 6 hours. 1,2-Dichloroethane was then distilled off under reduced pressure. Then 300 mL of methyl tert-butyl ether was added to dissolve the crude product and washed with water. The organic phase was separated off and the methyl tert-butyl ether was then removed under ...
Embodiment 2
[0034] Preparation of 3-(7-tert-butyl)-3-(2,3-dimethylphenyl)-2-oxo-2,3-dihydrobenzofuran-5-yl)propanoic acid-6-methyl Heptyl ester and 3-(7-(tert-butyl)-3-(3,4-dimethylphenyl)-2-oxo-2,3-dihydrobenzofuran-5-yl)propane A mixture of isooctyl esters.
[0035] In a four-neck round bottom 500mL flask equipped with a mechanical stirrer, a thermal controller and a reflux condenser, 140g (0.4 moles) of the product of formula (II-1) prepared in Example 1, and 104 grams of isooctyl alcohol (EXXAL8 isooctyl alcohol 0.8 mole from ExxonMobil Chemical), 40 grams of toluene and 2 grams of aluminum triisopropoxide were mixed. The reaction mixture was stirred and heated to 85°C for 5 hours under nitrogen atmosphere, when the reaction was complete, 8.8 grams of aqueous citric acid solution (50%) was added, stirring was continued for 20 minutes, then 180 g of water was added and stirred for 20 minutes at 75°C minute. The organic phase was separated, washed twice with brine, and dried over sod...
Embodiment 3
[0038] Preparation of octyl 3-(7-(tert-butyl)-3-(2,3-dimethylphenyl)-2-oxo-2,3-dihydrobenzofuran-5-yl)propanoate and 3 - Mixtures of octyl (7-(tert-butyl)-3-(3,4-dimethylphenyl)-2-oxo-2,3-dihydrobenzofuran-5-yl)propanoate.
[0039] The preparation method is basically the same as in Example 2, except that n-octanol is used to replace the isooctyl alcohol in Example 2. 180 g of Compound (I-2) was isolated as a pale yellow viscous liquid. Analytical data of the compound of formula (I-2): MS (m / z: 478.31), 1H NMR (chemical shift of methine is 4.8),
[0040]
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com