2'-deoxy-2'-fluoro-2'-methylnucleoside derivative as well as preparation method and application of derivative in pharmaceuticals
A technology of methyl nucleoside derivatives and derivatives is applied in the field of preparing anti-hepatitis C virus drugs, 2'-deoxy-2'-fluoro-2'-methyl nucleoside derivatives, and can solve the problem of dosage large, drug resistance, recurrent virus infection and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] (S)-2-(((S)-(((2R, 3R, 4R, 5R)-5-(4-methylamino-2-oxopyrimidin-1(2H)-yl)-4-fluoro -3-Hydroxy-4-methyl-tetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoic acid isopropyl ester (I-1)
[0084]
[0085] Step A
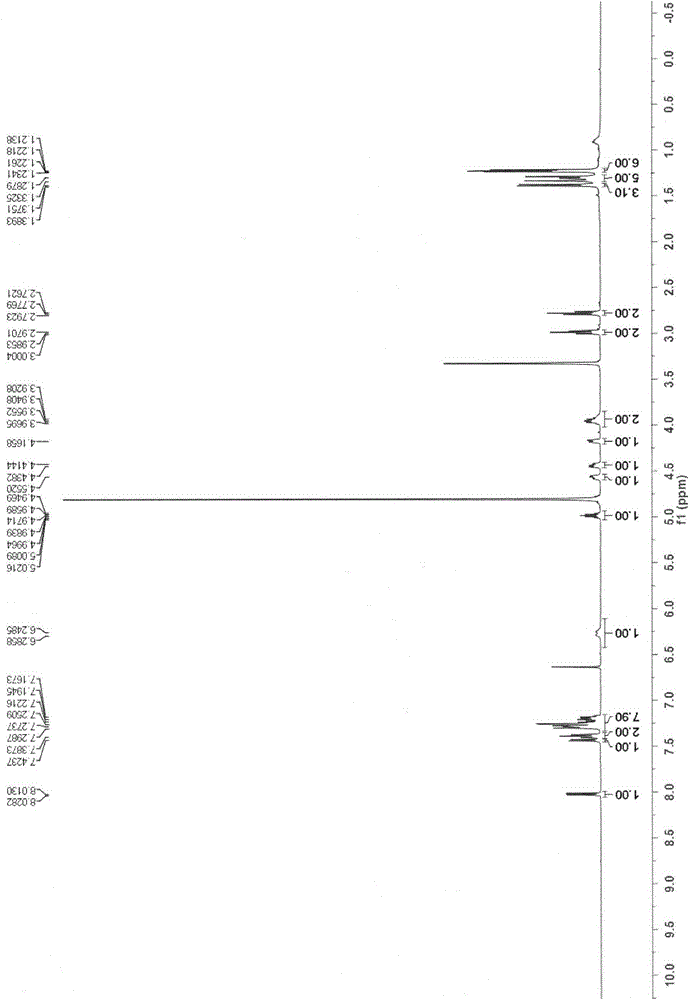
[0086] 3',5'-di-O-benzoyl-2'-deoxy-2'-fluoro-2'-methyluridine (II-1) (50 mg) was suspended in acetonitrile (4 ml), and three Ethylamine (30 mg) and 4-dimethylaminopyridine (40 mg) were stirred in an ice bath for 30 minutes, 2,4,6-triisopropylbenzenesulfonyl chloride (90 mg) was added, moved to room temperature and stirred for 24 hours, and methyl The tetrahydrofuran solution (2ml) of the amine was stirred overnight at room temperature; after concentrating part of the reaction solution, the residue was poured into water (20ml), and the aqueous phase was extracted with ethyl acetate (20ml x 3), and the organic phase was washed with saturated brine, without Dry over sodium sulfate, evaporate the organic phase to get the crude product, and get N4-methy...
Embodiment 2
[0092] (S)-2-(((S)-(((2R,3R,4R,5R)-5-(4-isopropylamino-2-oxopyrimidin-1(2H)-yl)-4- Fluoro-3-hydroxy-4-methyl-tetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoic acid isopropyl ester (I-2)
[0093]
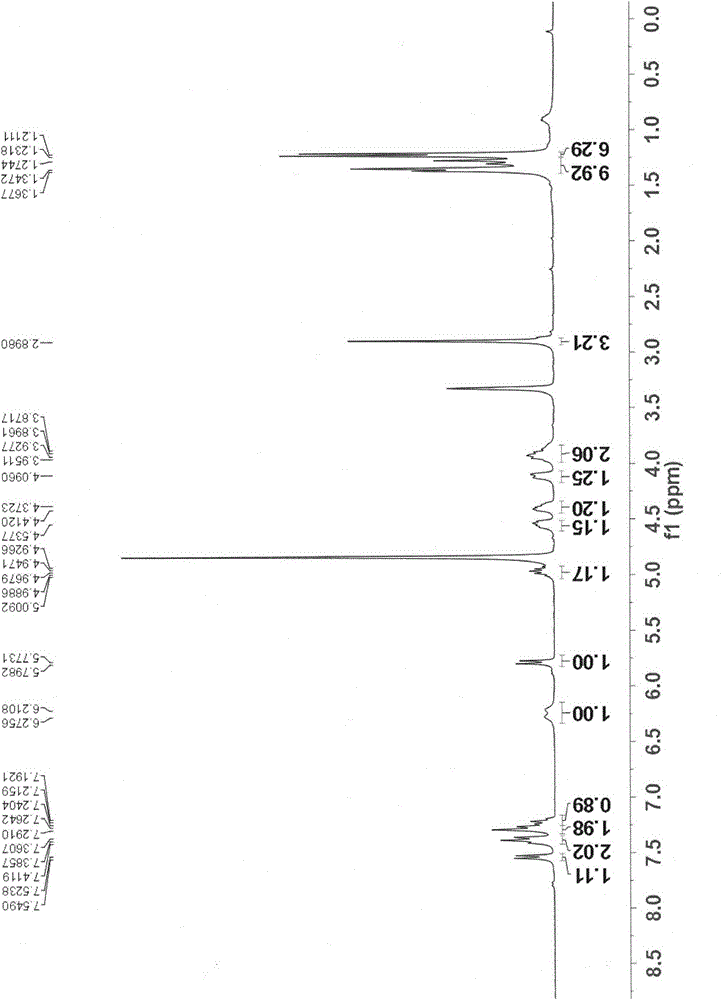
[0094] The preparation method refers to Example 1, replacing methylamine with isopropylamine to obtain compound I-2; 1 H NMR (300MHz, CD 3 OD) δ7.55(d, J=7.5Hz, 1H), 7.41(t, J=7.8Hz, 2H), 7.29(d, J=7.7Hz, 2H), 7.24(t, J=7.5Hz, 1H ), 6.25(br d, J=18.9Hz, 1H), 5.82(d, J=7.5Hz, 1H), 5.06-4.90(m, 1H), 4.65-4.44(m, 1H), 4.47-4.28(m , 1H), 4.12(br d, J=8.6Hz, 1H), 4.01-3.55(m, 4H), 1.36(d, J=5.2Hz, 3H), 1.31(d, J=21.5Hz, 3H), 1.29-1.07(m, 12H).
Embodiment 3
[0096] (S)-2-(((S)-(((2R,3R,4R,5R)-5-(4-n-butylamino-2-oxopyrimidin-1(2H)-yl)-4- Fluoro-3-hydroxy-4-methyl-tetrahydrofuran-2-yl)methoxy)(phenoxy)phosphoryl)amino)propanoic acid isopropyl ester (I-3)
[0097]
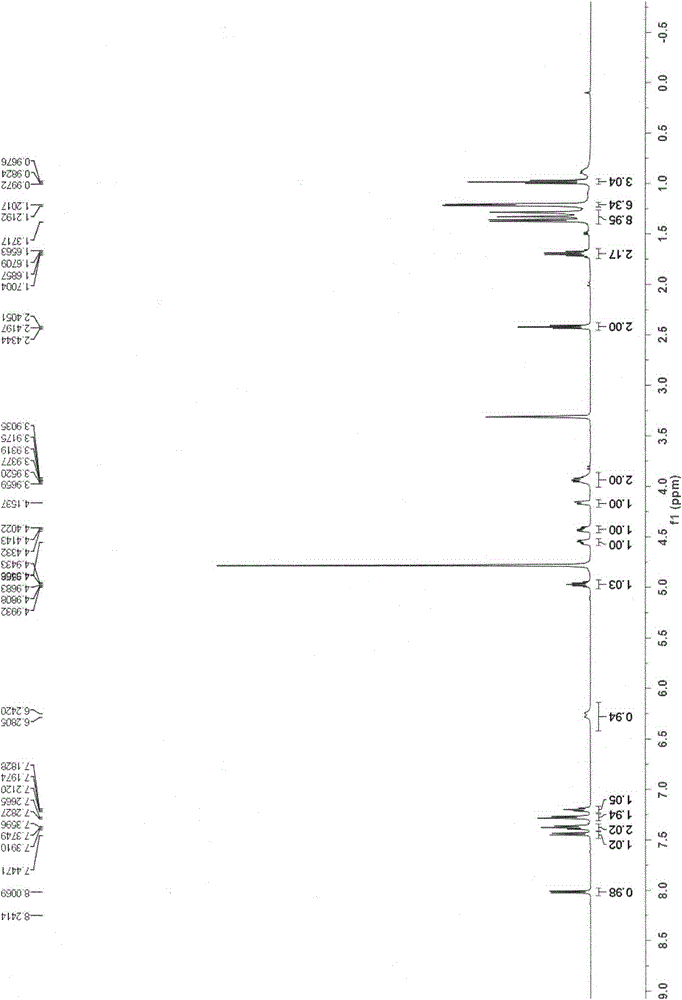
[0098] The preparation method refers to Example 1, and methylamine is replaced by n-butylamine to obtain compound I-3; 1 H NMR (300MHz, CD 3 OD) δ7.53(d, J=7.5Hz, 1H), 7.39(t, J=7.6Hz, 2H), 7.28(d, J=7.8Hz, 2H), 7.21(t, J=7.2Hz, 1H ), 6.24(br d, J=17.7Hz, 1H), 5.81(d, J=7.5Hz, 1H), 5.04-4.89(m, 1H), 4.66-4.49(m, 1H), 4.47-4.31(m , 1H), 4.11(br d, J=8.4Hz, 1H), 4.00-3.79(m, 2H), 3.38(t, J=6.9Hz, 2H), 1.67-1.50(m, 2H), 1.50-1.40 (m, 2H), 1.36(d, J=5.2Hz, 3H), 1.31(d, J=21.5Hz, 3H), 1.22(d, J=6.2Hz, 6H), 0.96(t, J=7.2Hz , 3H). 13 C NMR (75MHz, CD 3 OD) δ172.89 (d, J=5.4Hz), 163.76, 157.02, 150.74, 138.55 (br s), 129.42, 124.79, 119.93 (d, J=4.8Hz), 100.14 (d, J=181.3Hz), 96.22, 89.85(br s), 79.29(br s), 71.88(d, J=18.1Hz), 68.72, 64.48(br s), 50.23, 39.98, 30.63, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com