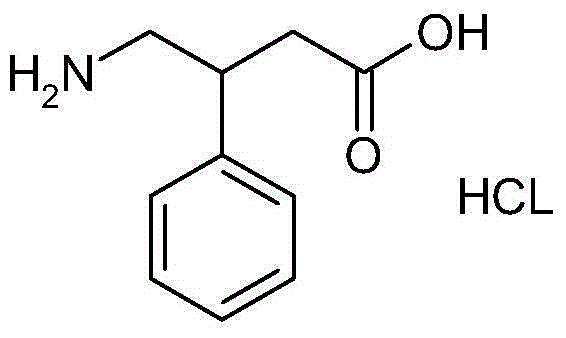
Synthetic method for 4-amino-3-phenylbutyric acid hydrochloride
A technology of phenylbutyrate hydrochloride and synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., and can solve problems such as high price of starting materials, heavy pollution in the production process, and cumbersome operation steps , to achieve the effect of low cost, high yield and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
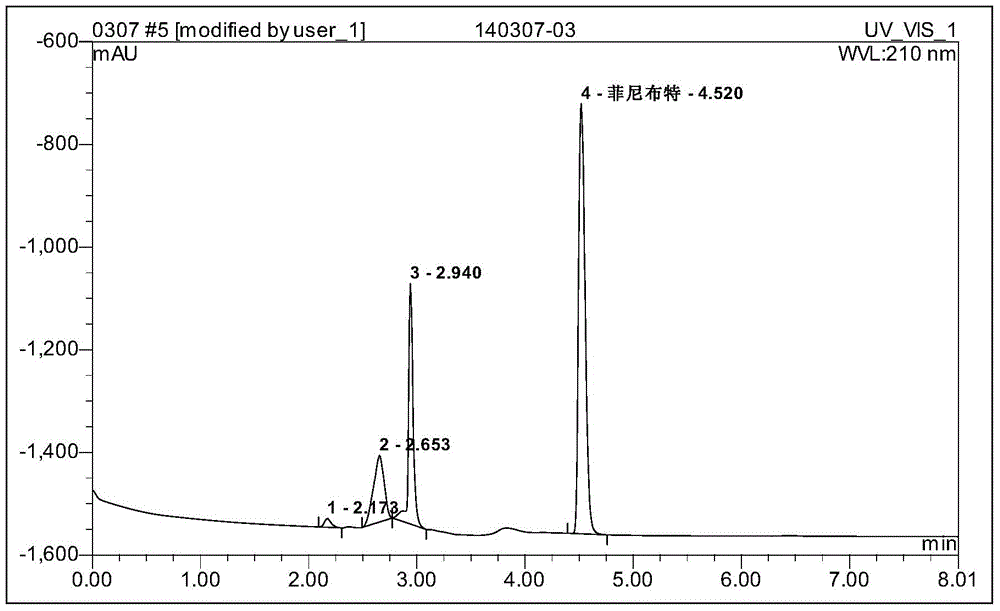
Examples
example 1
[0026] 1] Preparation of the first intermediate:
[0027] Add 265g (2.5mol) of benzaldehyde, 812.5g (6.25mol) of ethyl acetoacetate, and 20g of piperazine into 1000ml (97%) of ethanol, stir and react for 48 hours at room temperature 20°C-25°C, filter, rinse with absolute ethanol The filter cake was dried to obtain 700 g of solid, and the filtrate was concentrated to 1 / 4 of the original volume, cooled, filtered and washed with alcohol to obtain another 50 g of solid, which was combined twice for a total of 750 g, with a yield of 78%;
[0028] 2] Preparation of the second intermediate:
[0029] Add 280g (0.11mol) of the intermediate to 400mL of 20% sodium hydroxide solution (80g of sodium hydroxide dissolved in 320mL of water), stir at 85-90°C for 2-2.5h, filter, wash the filter cake with water, and combine Filtrate, cooling the filtrate, adding 170 mL of hydrochloric acid dropwise under stirring and cooling to adjust the pH to 1-2, stirring under cooling for 3 h, filtering, wa...
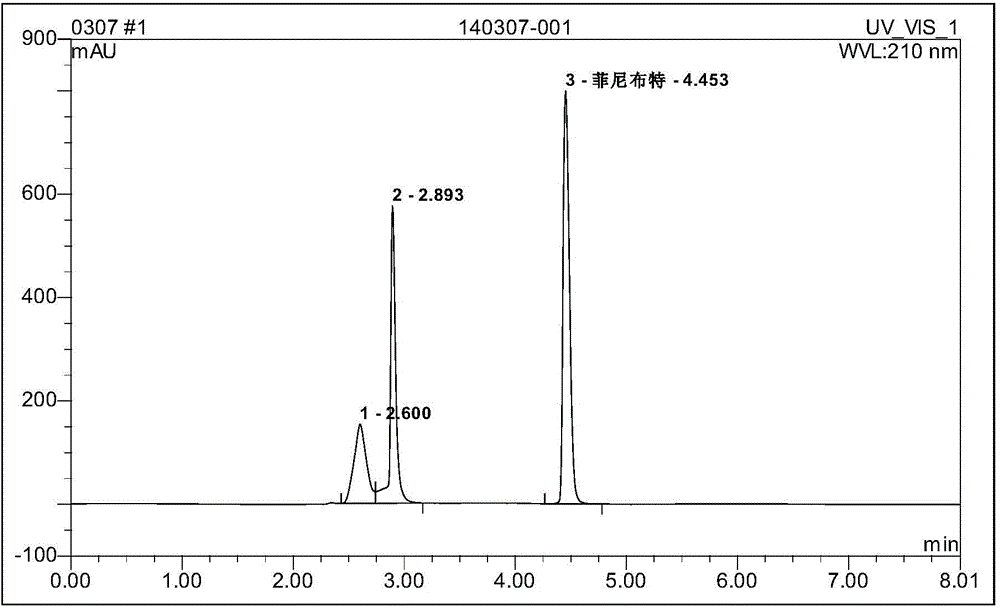
example 2
[0037] 1] Preparation of the first intermediate:
[0038] Add 53g (0.5mol) of benzaldehyde, 162.5g (1.25mol) of ethyl acetoacetate, and 25g of triethylamine into 200ml of 95% (97%) ethanol, stir and react for 48h at room temperature at 20°C-25°C, filter, and wash with absolute ethanol Rinse the filter cake and dry to obtain 140 g of solid, concentrate the filtrate to 1 / 4, cold analyze, filter and wash with alcohol to obtain 6 g of solid, and combine the two solids to obtain 146 g in total, with a yield of 83%;
[0039] 2] Preparation of the second intermediate:
[0040] Add 140g (0.11mol) of the intermediate to 400mL of 20% sodium hydroxide solution (80g sodium hydroxide dissolved in 320mL water), stir and react at 85-90°C for 2-2.5h, filter, wash the filter cake with water, combine the filtrates, and cool To the filtrate, 170 mL of HCl was added dropwise with stirring and cooling to adjust the pH to 1-2, and the mixture was stirred for 3 h under cooling, filtered, washed wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com