Transparent polyimide material and preparation method thereof
A transparent polyimide, polyimide technology, applied in the direction of organic chemistry, to achieve the effect of low water absorption, inhibit the formation of CTC, and increase transparency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of embodiment 1 compound 2-(3,5-dinitrophenyl)-4,5,6,7-(tetrafluoroisoindoline)-1,3-dione
[0035] 60.7g (0.255mol) of tetrafluorophthalic acid, 46.7g (0.255mol) of 3,5-dinitroaniline, and 600g of acetic acid were successively added into a 1L three-necked flask, and the reaction solution was yellow and turbid. Turn on the mechanical stirring, heat up to 125°C, reflux the system, the raw materials gradually dissolve, and become almost clear liquid. During the heat preservation and reflux process, the system slowly appeared yellow solid and turned into yellow turbidity, and the heat preservation reaction was 8h. The reaction solution was yellow and turbid, and was suction filtered at room temperature to obtain a yellow solid, rinsed with absolute ethanol once, the color was lighter, and a light yellow solid was obtained, which was 2-(3,5-dinitrophenyl)-4,5 , 6,7-(tetrafluoroisoindoline)-1,3-dione, dried in a vacuum oven at room temperature for 4 hours, we...
Embodiment 2
[0037]60.7g (0.255mol) of tetrafluorophthalic acid, 46.7g (0.255mol) of 3,5-dinitroaniline, and 600g of acetic acid were successively added into a 1L three-necked flask, and the reaction solution was yellow and turbid. Turn on the mechanical stirring, heat up to 130°C, reflux the system, the raw materials gradually dissolve, and become almost clear liquid. During the heat preservation and reflux process, the system slowly appeared yellow solid and turned into yellow turbidity, and the heat preservation reaction was carried out for 10 hours. The reaction solution was yellow and turbid, and was suction filtered at room temperature to obtain a yellow solid, rinsed with absolute ethanol once, the color was lighter, and a light yellow solid was obtained, which was 2-(3,5-dinitrophenyl)-4,5 , 6,7-(tetrafluoroisoindoline)-1,3-dione, dried in a vacuum oven at room temperature for 4.5h, weighed 78.2g, yield 79.1%.
Embodiment 3
[0039] 60.7g (0.255mol) of tetrafluorophthalic acid, 46.7g (0.255mol) of 3,5-dinitroaniline, and 600g of acetic acid were successively added into a 1L three-necked flask, and the reaction solution was yellow and turbid. Turn on the mechanical stirring, heat up to 127°C, reflux the system, the raw materials gradually dissolve, and become almost clear liquid. During the heat preservation and reflux process, the system slowly appeared yellow solid and turned into yellow turbidity, and the heat preservation reaction was 9h. The reaction solution was yellow and turbid, and was suction filtered at room temperature to obtain a yellow solid, rinsed with absolute ethanol once, the color was lighter, and a light yellow solid was obtained, which was 2-(3,5-dinitrophenyl)-4,5 , 6,7-(tetrafluoroisoindoline)-1,3-dione, dried in a vacuum oven at room temperature for 4 hours, weighing 78g, yield 79%.
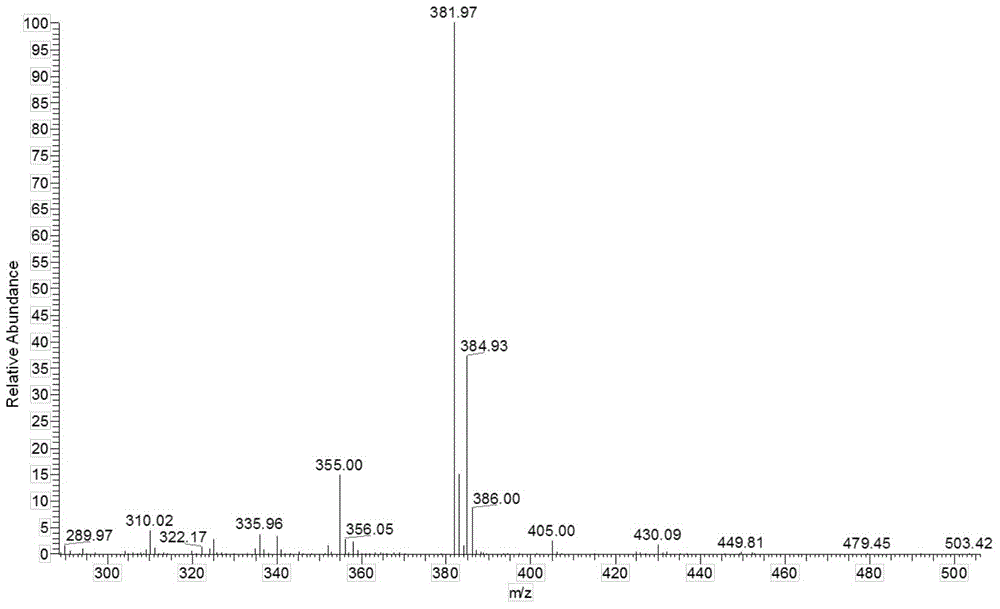
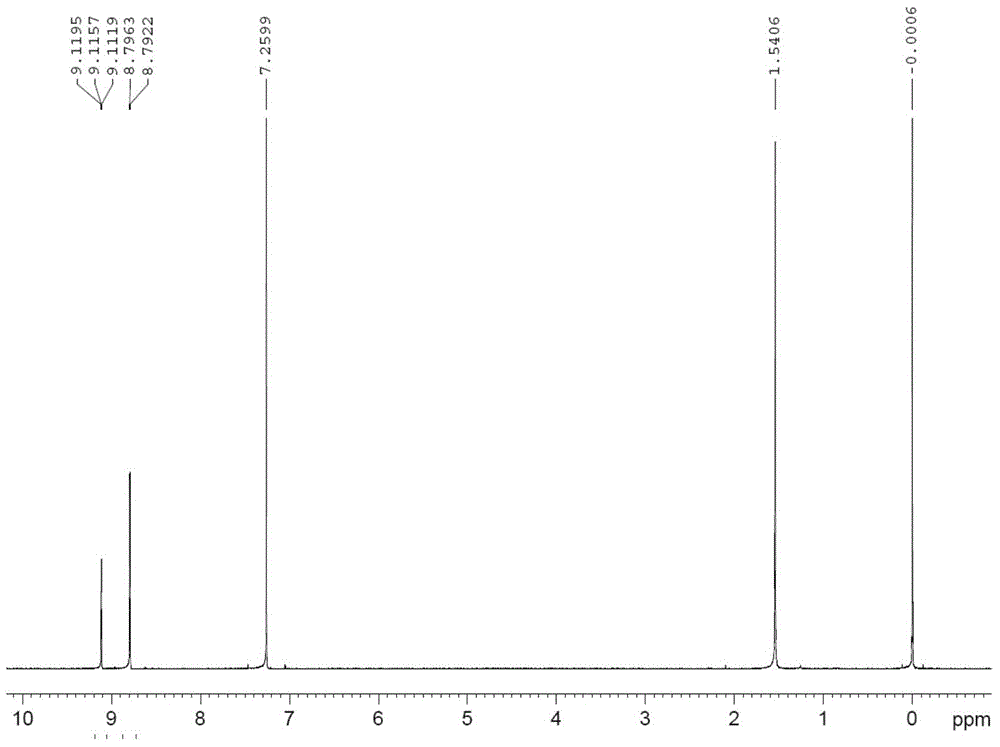
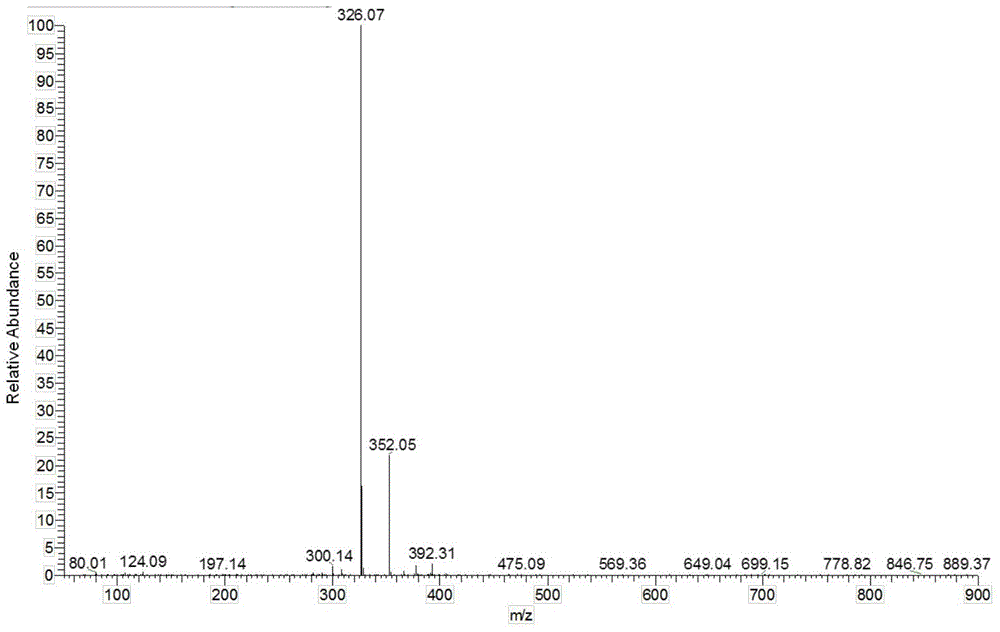
[0040] In Example 1-3, the characterization data of the prepared 2-(3,5-dinitrophenyl)-4,5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com