A stable liquid pharmaceutical composition containing animal dander allergen
A technology of animal dander and liquid medicine, which is applied in the direction of drug combination, allergen antigen components, allergic diseases, etc., can solve the problems of short shelf life and achieve high stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Example 1: Preparation of liquid pharmaceutical composition containing cat dander allergen
[0078] Select the cat dander allergen extract as the allergen to prepare a stable liquid pharmaceutical composition, and the specific steps are as follows:
[0079] 1. Crushing: liquid nitrogen crushes the raw materials of Chinese pastoral cat dander;
[0080] 2. Degreasing: Add acetone to degrease according to the mass volume ratio of about 1:10, stir for about 2 hours, filter, discard the acetone solution, and degrease for a total of 3 times. The degreasing liquid is basically colorless by visual inspection. The degreasing powder is dried for no less than 36 hours until there is no smell of acetone.
[0081] 3. Extraction: Add 50 mM pH 8.2 phosphate buffer containing 1.0% NaCl at 2-8° C. for an extraction ratio of 1:20 (w / v), and extract for 48-60 hours. Centrifuge and filter step by step until the 0.22μm membrane filter is smooth.
[0082] 4. Concentration: Divide the cru...
Embodiment 2
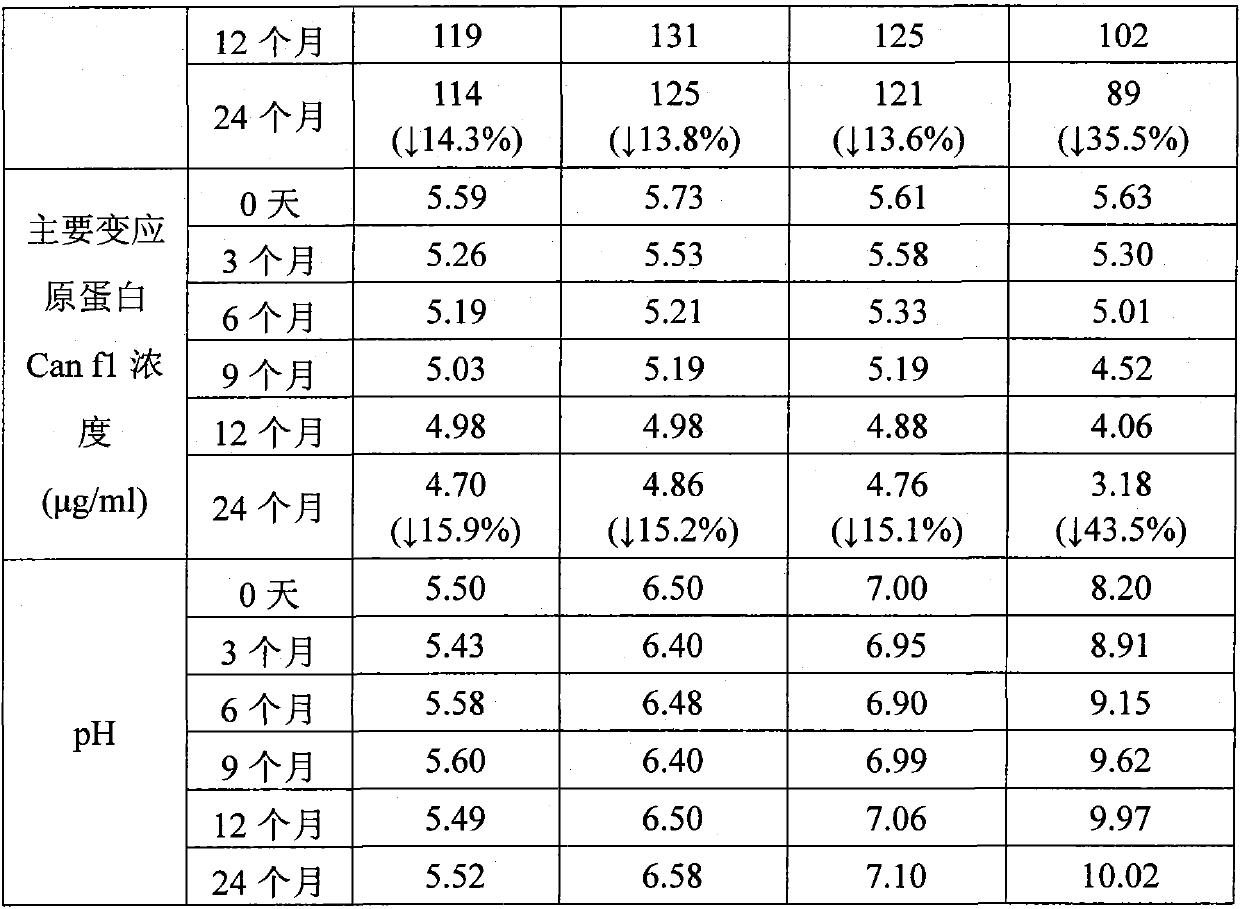
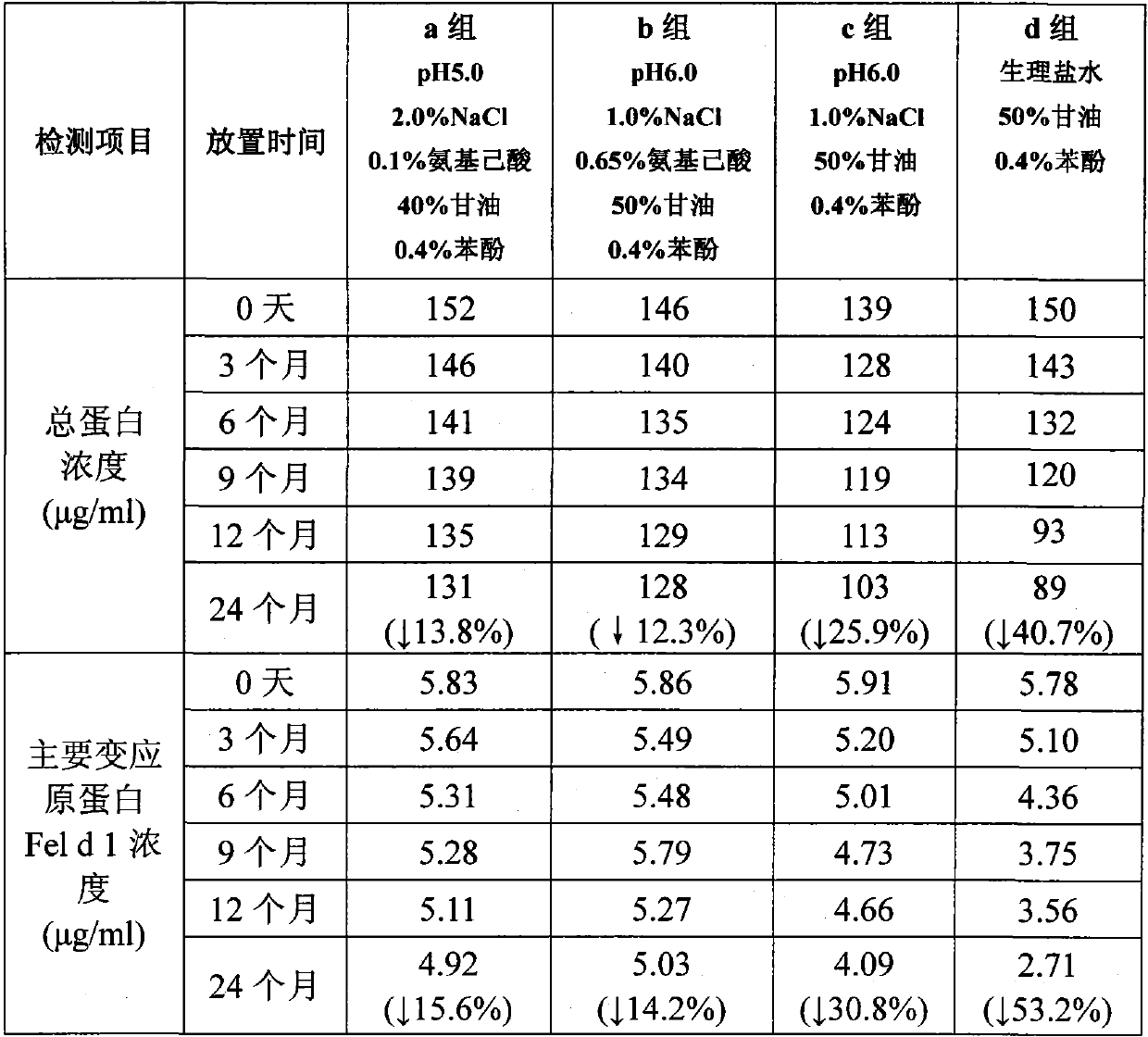
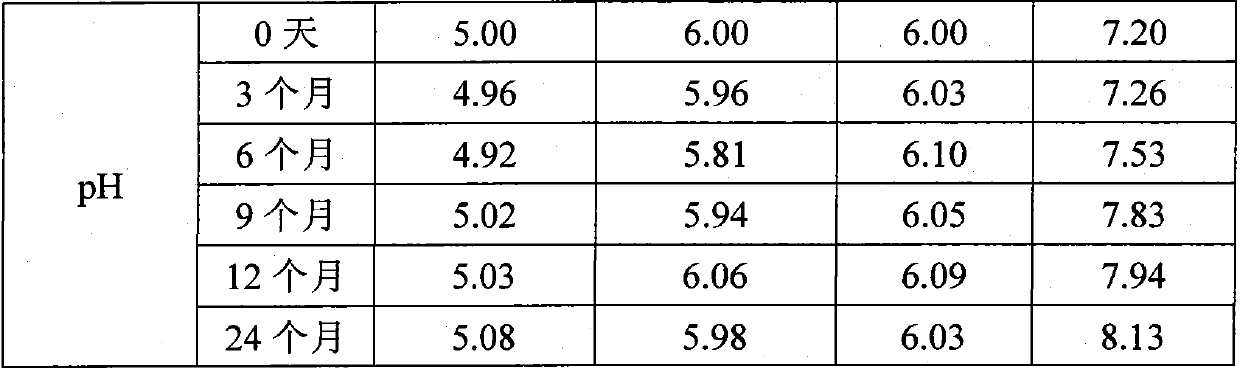
[0089] Example 2: Long-term stability test of liquid pharmaceutical composition containing cat dander allergen at 2-8°C
[0090] Four groups of liquid pharmaceutical compositions containing cat dander allergens were respectively prepared according to the four proportions of a, b, c, and d in Example 1 of the present invention. 0 month, 3 months, 6 months, 9 months, 12 months, and 24 months were tested. The total protein concentration was detected by Brandford method, and the main allergen protein Fel d 1 concentration was detected by ELISA method. The results are shown in Table 1:
[0091] Table 1
[0092]
[0093]
[0094] The test results showed that under the condition of 2-8°C, by the end of 24 months, the total protein concentration of the liquid pharmaceutical composition of group a decreased by 13.8%, and the main allergen protein concentration decreased by 15.6%; The protein concentration decreased by 12.3%, and the major allergen protein concentration decreas...
Embodiment 3
[0096] Example 3: Stability test of liquid pharmaceutical composition containing cat dander allergen at 25°C±2°C
[0097] Four groups of liquid pharmaceutical compositions containing cat dander allergen were respectively prepared according to the four proportions of a, b, c, and d in Example 1 of the present invention, which were placed under the condition of 25°C±2°C, and respectively At the end of the 0th month, 1st month, 2nd month, 3rd month and 6th month. The total protein concentration was detected by Brandford method, and the main allergen protein Fel d 1 concentration was detected by ELISA method. The results are shown in Table 2:
[0098] Table 2
[0099]
[0100]
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com