Detection kit and detection method for pkd1 gene mutation
A kit and gene technology, applied in the field of medical molecular biology detection, can solve problems such as low efficiency, poor sequencing quality, and unsatisfactory primer stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Example 1: Using the true gene-specific primers (E2, T3, K3, R5, K5) of the PKD1 gene exon 2-46 region to perform long-fragment PCR and high-throughput sequencing to detect PKD1 gene mutations
[0087] On the premise of obtaining the informed consent of the subjects, the PKD1 gene mutation was detected by long-fragment PCR combined with high-throughput sequencing for 70 subjects.
[0088] Use the OMEGA Genomic DNA Extraction Kit (purchased from OMEGA, USA) to extract the genomic DNA from the peripheral blood of the subject, and use a spectrophotometer or other testing instruments to detect the DNA concentration and purity of the extracted DNA. The DNA concentration is greater than 50 ng / μl, and the volume is greater than 30μl, A260 / A280 between 1.6-2.0, as a DNA template. The DNA templates from each subject were equally divided into five separate reaction tubes, and long-segment PCR primers E2, T3, K3, R5, and K5 (sequences are shown in Table 1) were added to each of th...
Embodiment 2
[0141] Embodiment 2: The primer specificity comparison of the method of PKD1 gene-specific T3 primer (SEQ ID NO:3 and 4) and R5 primer (SEQ ID NO:7 and 8) and Adrian Y.Tan etc.
[0142] On the premise of obtaining the informed consent of the subject, peripheral blood was collected from one subject, and the genomic DNA of the subject's peripheral blood was extracted with the OMEGA Genomic DNA Extraction Kit (purchased from OMEGA Company, USA). Spectrophotometer or other detection equipment to detect DNA concentration and purity, DNA concentration greater than 50ng / μl, volume greater than 30μl, A260 / A280 between 1.6-2.0, used as template DNA.
[0143] Using primers PKD1_NGS_2-12F, PKD1_NGS_2-12R, PKD1_NGS_13-21F, PKD1_NGS_13-21R, PKD1_NGS_22-34F, PKD1_NGS_22-34R and its PCR reaction system and PCR reaction conditions to amplify exon 2-34 according to the method of Adrian Y.Tan et al. In addition, E2 primers (SEQ ID NOs: 1 and 2), T3 primers (SEQ ID NOs: 3 and 4), K3 primers (SEQ...
Embodiment 3
[0150] Embodiment 3: The primer stability comparison of E2, T2, R5 primer and the method such as Adrian Y.Tan
[0151] On the premise of obtaining the informed consent of the subjects, peripheral blood was collected from 8 subjects, and the genomic DNA of the subjects' peripheral blood was extracted with the OMEGA Genomic DNA Extraction Kit (purchased from OMEGA Company, USA). Spectrophotometer or other detection equipment to detect DNA concentration and purity, DNA concentration greater than 50ng / μl, volume greater than 30μl, A260 / A280 between 1.6-2.0, used as template DNA.
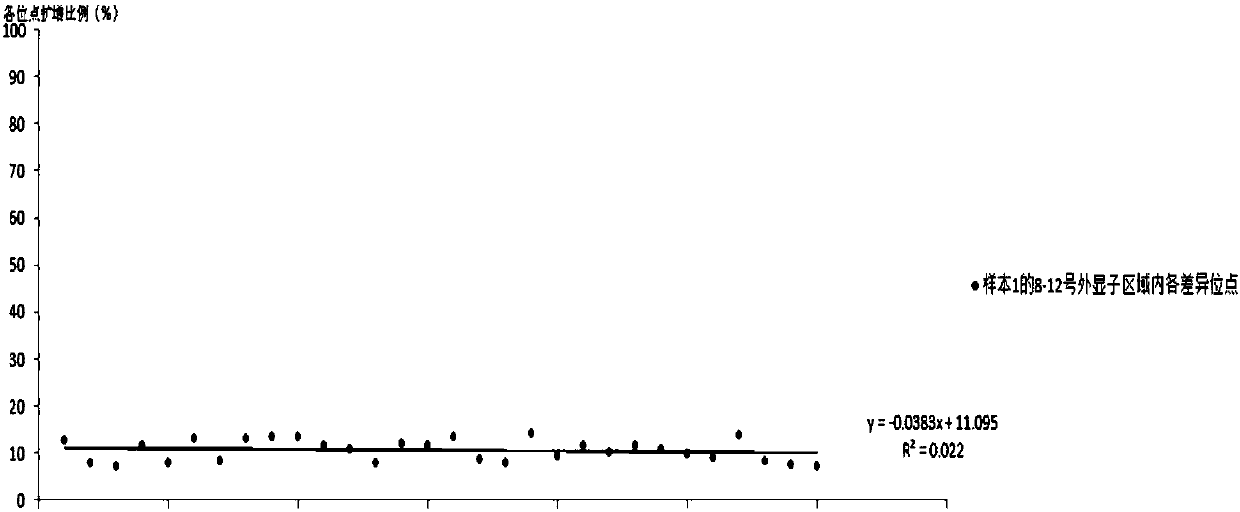
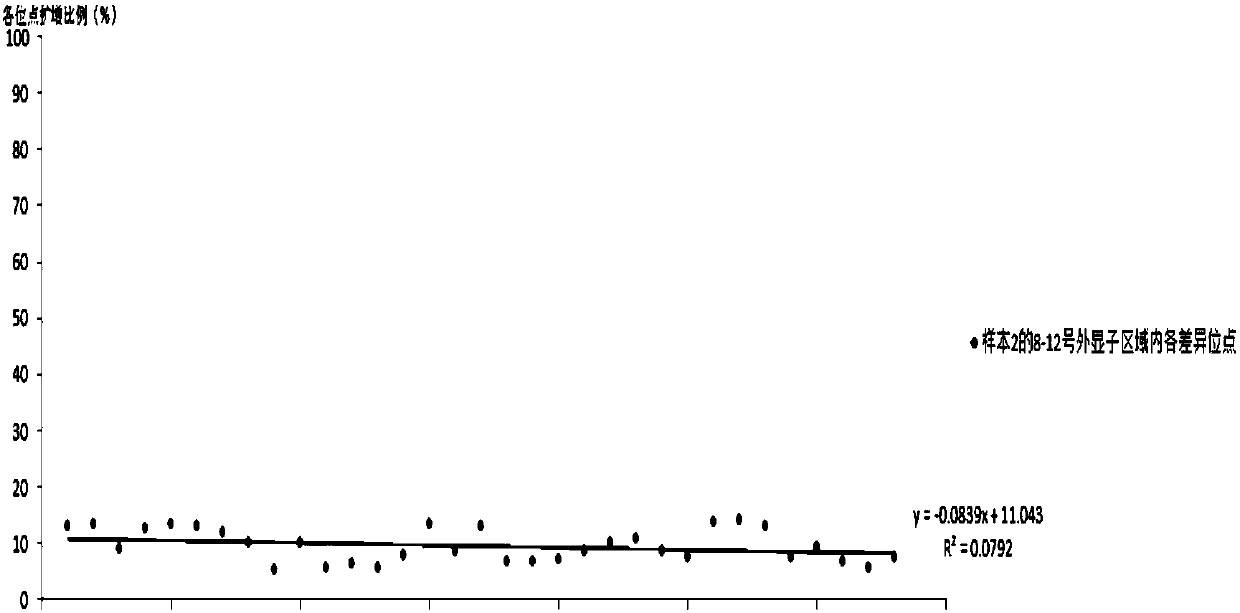
[0152] The primers PKD1_NGS_2-12F, PKD1_NGS_2-12R of the method such as Adrian Y.Tan, PKD1_NGS_2-12R and its PCR reaction system and PCR reaction conditions, adopt the E2 primer (SEQ ID NO:1 and 2) primers and T3 primers (SEQ ID NO:3 and 4) primers and the reaction system described in the above table 2 and the PCR reaction conditions described in the above table 3 for amplifying No. 8-12 exon regions, re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com