Method for rapidly evaluating activity of catalyst for cyclic ester ring opening polymerization in industrial production process
A technology of ring-opening polymerization and catalyst, applied in the field of polyester materials, can solve the problems of cumbersome steps and high cost, and achieve the effects of reliable test data, good reproducibility and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
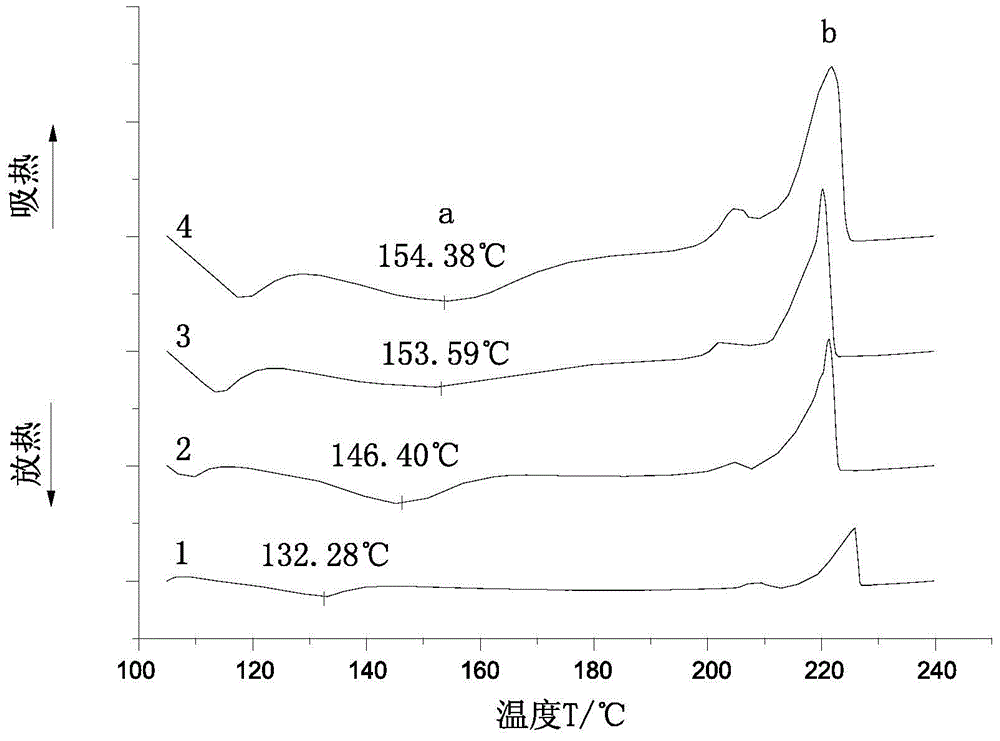
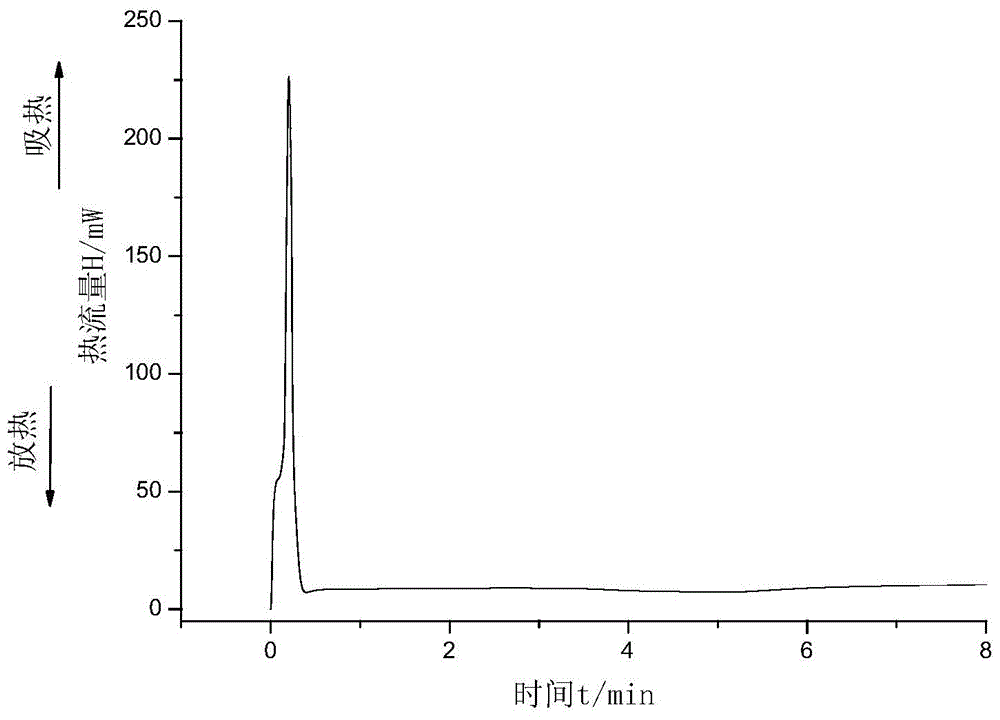
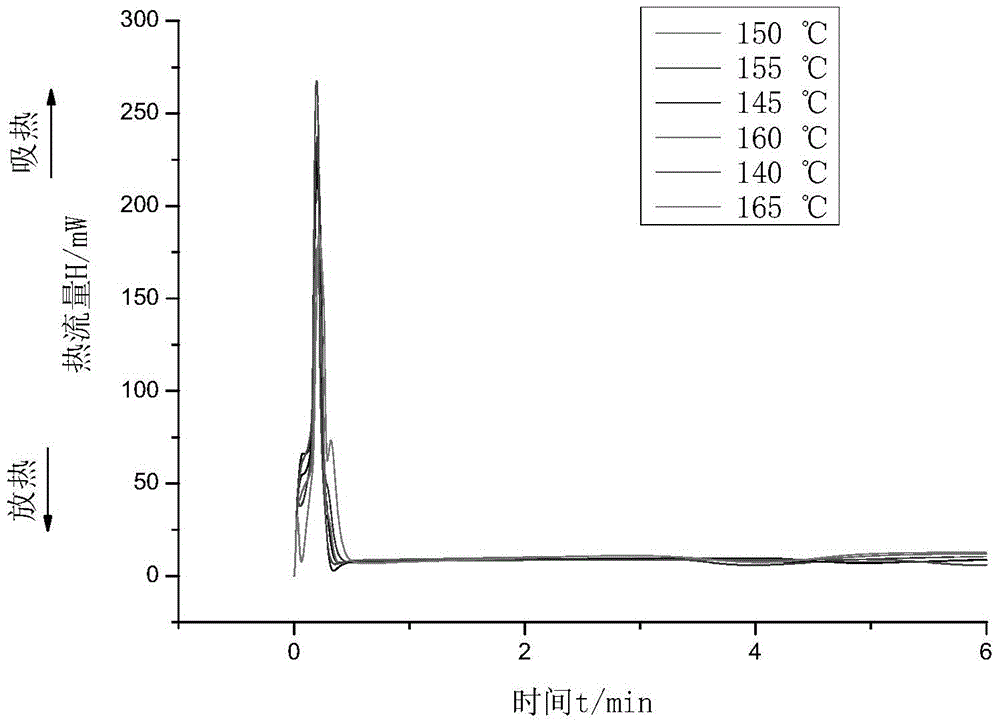
[0039] 5 g of monomer and catalyst were used and mixed under vigorous stirring. After that, an appropriate amount of sample was taken for DSC measurement. DSC experiments were performed on a PerkinElmer 8500DSC instrument equipped with a cooling system. The most typical sample size for DSC measurements is 3-5 mg. The flow rate of nitrogen into the DSC sample cell is 50mL min -1 . The experiments were carried out in sealed aluminum crucibles. The relevant data were analyzed using Pyris V11 Data Analysis software.
[0040] First, the DSC reactants were completely sealed at 5 and 7.5 °C min-1 The heating rate was raised in the temperature range of 30 to 250 ° C, and the kinetic parameters of the polymerization at a uniform temperature were measured. The results are shown in Table 1:
[0041] Table 1: Temperature sweep kinetic parameters of GA homopolymerization at 145°C
[0042]
[0043] Note: catalyst, co-catalyst and initiator dosage ratio (molar ratio) are
[0044] n...
Embodiment 2
[0052] Except that the catalyst was changed to Bi(OAc) 3 Except its composite catalyst, adopt same measuring method as embodiment 1. The operating conditions and results are shown in Tables 3 and 4.
[0053] Table 3: Non-isothermal kinetic parameters of GA homopolymerization at 145 °C
[0054]
[0055] Note: the catalyst, co-catalyst and initiator consumption ratio (molar ratio) of each item in the above table are in line with:
[0056] no GA :n Bi(OAc)3 = 100; n Ph3P :n Bi(OAc)3 = 1; n MeOH :n Bi(OAc)3 =0.5
[0057] Table 4: Isothermal kinetic parameters of GA homopolymerization at 145 °C
[0058]
[0059] Note: the catalyst, co-catalyst and initiator consumption ratio (molar ratio) of each item in the above table are in line with:
[0060] no GA :n Bi(OAc)3 = 100; n Ph3P :n Bi(OAc)3 = 1; n MeOH :n Bi(OAc)3 =0.5
[0061] As can be seen from the data in Table 3 and Table 4, to Bi(OAc) 3 Add Ph to the system 3 After P and MeOH, the activation energy of ...
Embodiment 3
[0063] In addition to changing the catalyst to SnCl 2 2H 2 Except for O and its composite catalyst, the same measuring method as in Example 1 was adopted. The operating conditions and results are shown in Tables 5 and 6.
[0064] Table 5: Non-isothermal kinetic parameters of GA homopolymerization at 145 °C
[0065]
[0066] Note: the catalyst, co-catalyst and initiator consumption ratio (molar ratio) of each item in the above table are in line with:
[0067] no GA :n SnCl2·2H2O = 100; n Ph3P :n SnCl2·2H2O = 1; n MeOH :n SnCl2·2H2O =0.5
[0068] Table 6: Isothermal kinetic parameters of GA homopolymerization at 145 °C
[0069]
[0070] Note: the catalyst, co-catalyst and initiator consumption ratio (molar ratio) of each item in the above table are in line with:
[0071] no GA :n SnCl2·2H2O = 100; n Ph3P :n SnCl2·2H2O = 1; n MeOH :n SnCl2·2H2O =0.5
[0072] in Bi(OAc) 3 In the reaction of catalyzing the ring-opening polymerization of GA, at 145°C, E a 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com