Polar-reversible transformation solvent for isobutylene hydroformylation reaction
An isobutylene hydroformyl and reversible technology, applied in the chemical industry, can solve the problems of volatile solvent loss and high energy consumption, and achieve the effect of reducing energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
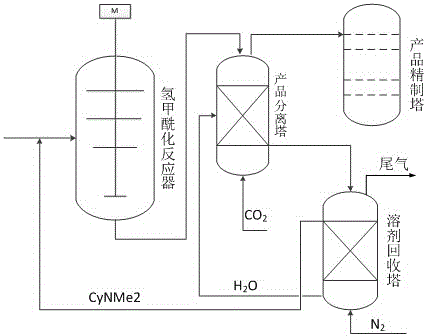
[0018] In a 250mL autoclave, add about 0.2g triphenylphosphinecarbonyl rhodium catalyst and 90mL CyNMe 2 As a solvent, the N 2 After replacement, fill in about 45g of isobutene, and then fill in a certain amount of mixed gas (CO / H 2 Volume ratio, 55:45), the temperature was raised to 100°C, and the reaction was carried out at 2.5MPa for 2h, and the rotation speed was 350rpm. After the reaction, the system was cooled to room temperature, about 100 mL of water was added to the system, and CO was passed at a flow rate of 200 mL / min. 2 After 30min, the solvent entered into the aqueous phase and separated from the organic phase of the product. The product phase is further separated and purified, and the yield of the obtained isovaleraldehyde is 70%, and the purity of the isovaleraldehyde product is 98%, wherein the nitrogen content is lower than 0.1%. The water phase was passed through N at a flow rate of 200mL / min 2 1 h, solvent CyNMe 2 It is dissociated from the water phase...
Embodiment 2
[0020] In a 250mL autoclave, add about 0.2g triphenylphosphinecarbonylrhodium catalyst and 100mL CyNMe 2 As a solvent, the N 2 After replacement, fill in about 45g of isobutene, and then fill in a certain amount of mixed gas (CO / H 2 Volume ratio, 55:45), the temperature was raised to 100°C, and the reaction was carried out at 2.5MPa for 2h, and the rotation speed was 350rpm. After the reaction, the system was cooled to room temperature, about 100 mL of water was added to the system, and CO was passed at a flow rate of 200 mL / min. 2 After 20min, the solvent enters the aqueous phase and separates the organic phase of the product. The product phase is further separated and purified, and the yield of isovaleraldehyde obtained is 65%, and the purity of the isovaleraldehyde product is 95%, wherein the nitrogen content is 2%. The water phase was passed through N at a flow rate of 200mL / min 2 1 h, solvent CyNMe 2 It is dissociated from the water phase and used for the next reac...
Embodiment 3
[0022] In the autoclave of 500mL, add about 0.35g triphenylphosphine carbonyl rhodium catalyst and 170mLCyNMe 2 As a solvent, the N 2 Fill in about 80g of isobutene after replacement, and then fill in a certain amount of mixed gas (CO / H 2 , 55:45), the temperature was raised to 100°C, and the reaction was carried out at 2.5MPa for 2h with a rotation speed of 350rpm. After the reaction, the system was cooled to room temperature, about 200 mL of water was added to the system, and CO was passed at a flow rate of 200 mL / min. 2 After 50min, the solvent entered into the aqueous phase and separated from the organic phase of the product. The product phase is further separated and purified, and the yield of the obtained isovaleraldehyde is 63%, and the purity of the isovaleraldehyde product is 98%, wherein the nitrogen content is lower than 0.1%. The water phase was passed through N at a flow rate of 200mL / min 2 2h, solvent CyNMe 2 It is dissociated from the water phase and used ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com