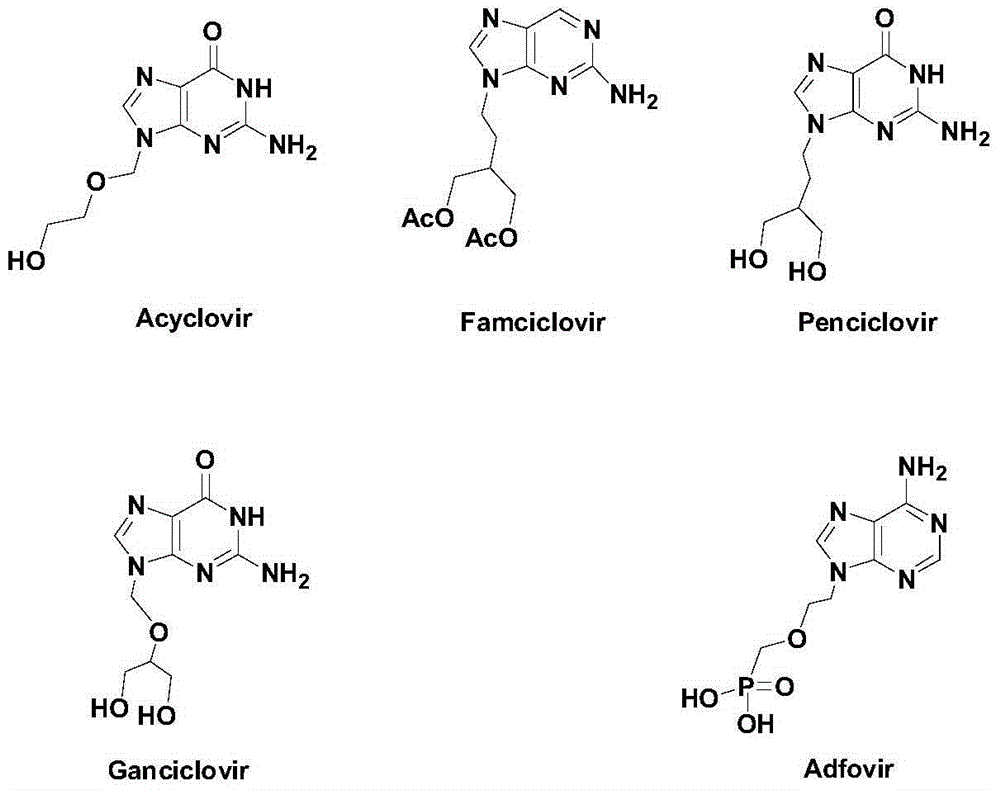
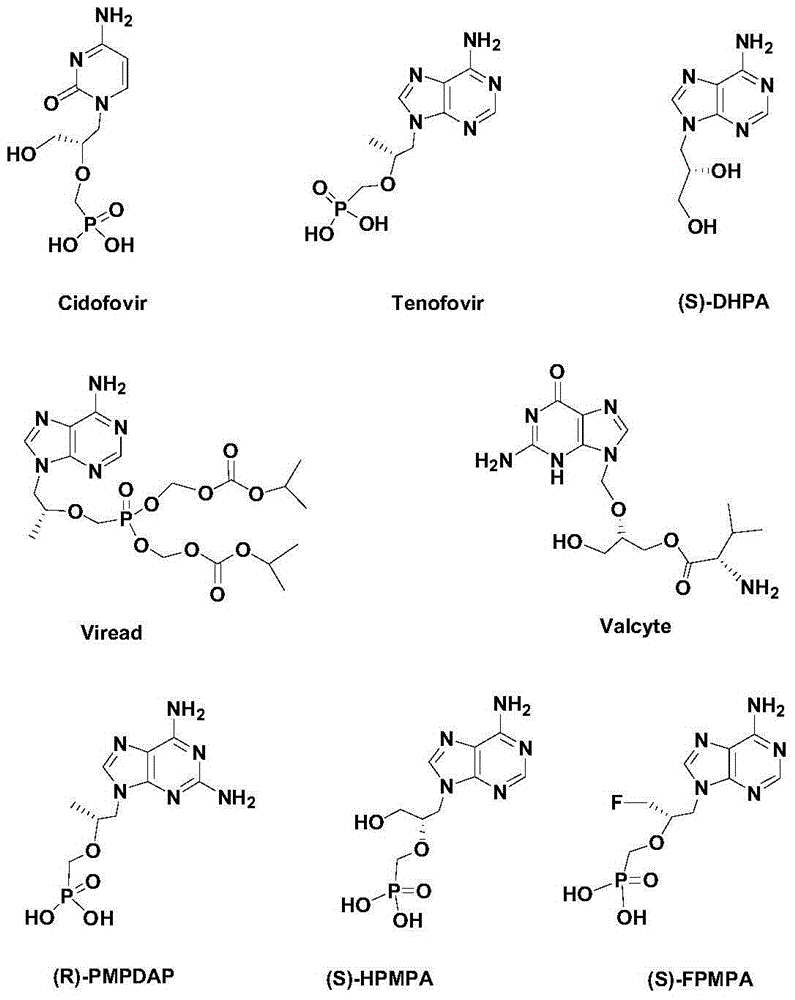
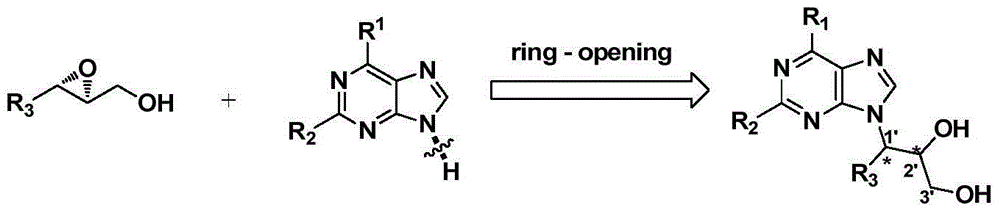
Synthetic method of novel chiral non-cycle purine nucleoside analogue
A cyclic purine nucleoside and a synthesis method technology are applied in the field of synthesis of novel chiral acyclic purine nucleoside analogs, and can solve the problems of single structure, difficult to obtain chiral source, many reaction steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] Dissolve 0.1mmol of adenine and 0.05mmol of t-BuOK in 0.5mL of DMF in a reaction vessel, stir at room temperature for 2 hours, then add 0.11moL of epoxy cinnamyl alcohol, stir and react at 80°C for 12 hours, and obtain Chiral acyclic nucleoside analog, white solid, yield 90%, ee>99%. 1 H NMR (DMSO-d 6 ,400MHz)δ8.46(s,1H),8.12(s,1H),7.48(d,J=6.8,1H),7.33-7.25(M,5H),5.76(d,J=5.6Hz,1H) ,5.57(d,J=5.2Hz,1H),4.89(q,J=5.4Hz,1H),4.46-4.40(m,1H),3.33-3.28(m,1H),3.24-3.18(m,1H ) 13 CNMR (DMSO-d 6 , 100MHz) δ156.4, 152.8, 149.6, 140.5, 137.8, 129.2, 128.6, 128.1, 118.8, 72.4, 63.1, 59.4, HRMS: calcd for C 14 h 16 N 5 o 2 [M+H + ]286.1299, found 286.1293.HPLC DAICEL CHIRALCEL OJ-H, hexane / iPOH=90 / 10, λ=254nm; retention time: 43.196min(minor), 49.238min(major), 99%ee.flow rate 1.0mL / min .
Embodiment 2
[0034] The base t-BuOK in Example 1 was replaced by NaH, and other conditions remained unchanged, the yield of chiral acyclic nucleoside analogs was 88%, and the ee value was 99%.
Embodiment 3
[0036] The reaction temperature in Example 1 was changed to 110° C., and the other conditions remained unchanged, and the yield of chiral acyclic nucleoside analogs was 93%, and the ee value was 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com