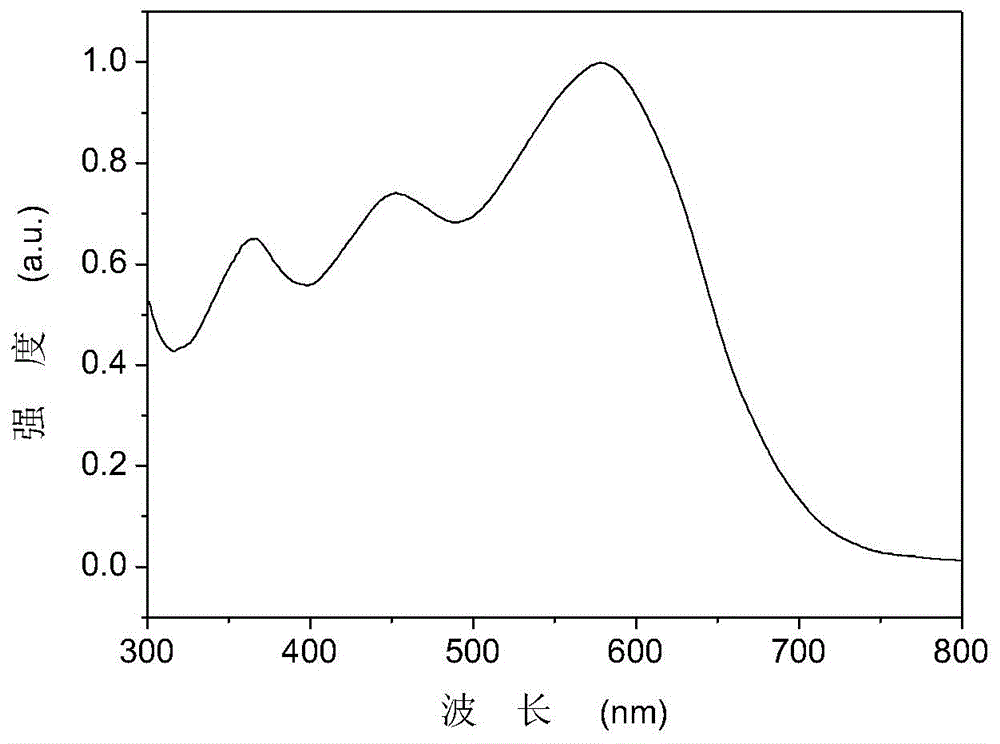
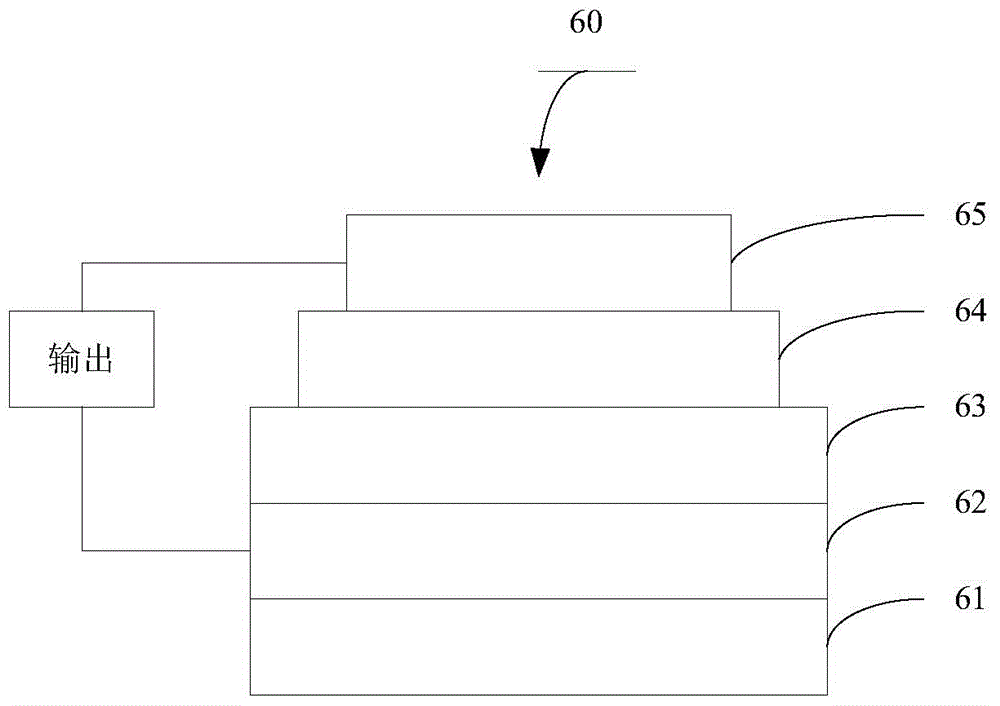
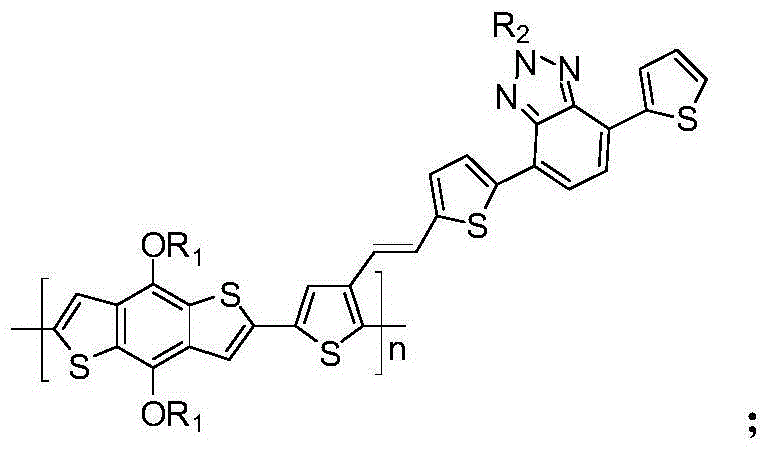
Triphenylamine side chain-containing polymer, preparation method thereof and organic solar cell device
A technology of triphenylamine and polymers, which is applied in the field of organic solar cell devices, polymers containing triphenylamine side chains and its preparation, can solve the problems of low conversion efficiency, improve energy conversion efficiency, novel structure, and improve low efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The polymer containing triphenylamine side chains in this example, that is, poly{2,6-diyl-4,8-bis(n-octyloxy)benzodithiophene-co-4-(2-(2,5 -diylthiophen-3-yl)vinyl)-N-(4-(n-octyloxy)benzene)-N-phenylaniline} (P1), (where R1 is n-octyl, R2 is n- Octyl, n=50), its structural formula is as follows:
[0032]
[0033] The preparation steps of above-mentioned polymer are as follows:
[0034] The reaction formula is as follows:
[0035]
[0036] Under the protection of argon, 2,6-ditrimethyltin-4,8-bis(n-octyloxy)benzodithiophene (151mg, 0.2mmol), 4-(2-(2,5-bis Bromothien-3-yl) vinyl)-N-(4-(n-octyloxy)benzene)-N-phenylaniline (128mg, 0.2mmol) was added to a flask containing 10ml of toluene solvent, and vacuumed to remove oxygen And filled with argon, then added bistriphenylphosphine palladium dichloride (5.6mg, 0.008mmol); the flask was heated to 100°C for Stille coupling reaction for 36h. Subsequently, the polymerization reaction was stopped after cooling down, and ...
Embodiment 2
[0040] The polymer containing triphenylamine side chains in this example, that is, poly{2,6-diyl-4,8-di(methoxy)benzodithiophene-co-4-(2-(2,5- Diylthiophen-3-yl)vinyl)-N-(4-(n-eicosyloxy)benzene)-N-phenylaniline} (P2), (where R1 is methyl, R2 is di Decyl, n=35), its structural formula is as follows:
[0041]
[0042] The preparation steps of above-mentioned polymer are as follows:
[0043] The reaction formula is as follows:
[0044]
[0045] Under the protection of mixed gas of nitrogen and argon, 2,6-ditrimethyltin-4,8-di(methoxy)benzodithiophene (173mg, 0.3mmol), 4-(2-(2,5 -Dibromothiophen-3-yl)vinyl)-N-(4-(n-eicosyloxy)benzene)-N-phenylaniline (242mg, 0.3mmol) and 15mL tetrahydrofuran were added to a 50mL two-necked flask After fully dissolving, pass the mixed gas of nitrogen and argon to exhaust the air for about 20 minutes, then add tetrakistriphenylphosphine palladium (4mg, 0.003mmol) into it, and then fully pass the mixed gas of nitrogen and argon to exhaust t...
Embodiment 3
[0048] The polymer containing triphenylamine side chains in this embodiment, that is, poly{2,6-diyl-4,8-bis(n-eicosyloxy)benzodithiophene-co-4-(2-(2 ,5-diylthiophen-3-yl)vinyl)-N-(4-(methoxy)benzene)-N-phenylaniline} (P3), (where R1 is n-eicosyl, R2 is methyl, n=10), its structural formula is as follows:
[0049]
[0050] The preparation steps of above-mentioned polymer are as follows:
[0051] The reaction formula is as follows:
[0052]
[0053] Under nitrogen protection, 2,6-ditrimethyltin-4,8-bis(n-eicosyloxy)benzodithiophene (332mg, 0.3mmol), 4-(2-(2,5-bis Bromothien-3-yl)vinyl)-N-(4-(methoxy)benzene)-N-phenylaniline (179mg, 0.33mmol), palladium acetate (3.5mg, 0.015mmol) and tris(o-methyl Phenylphenyl)phosphine (21mg, 0.06mmol) was added to a flask containing 12mL of N,N-dimethylformamide, and then the flask was purged with nitrogen for about 20min; the flask was heated to 130°C for Stille Coupling reaction 6h. Subsequently, stop the polymerization reaction af...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com