Benzothiazolyl copolymer as well as preparation method and application thereof
A thiazolyl-based copolymer technology, applied in the field of solar cell materials, can solve the problems of spectral response mismatch, low carrier mobility, low carrier electrode collection efficiency, etc., and achieve good solubility, novel structure, Excellent film-forming performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
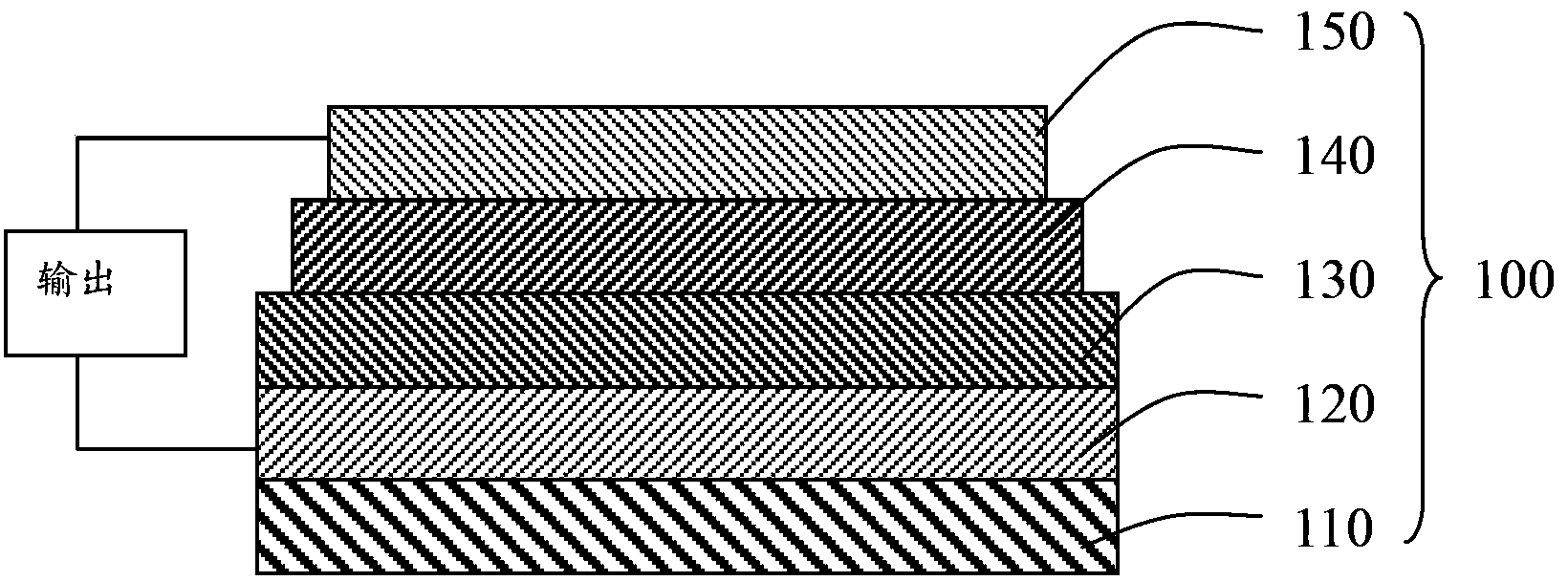
Image
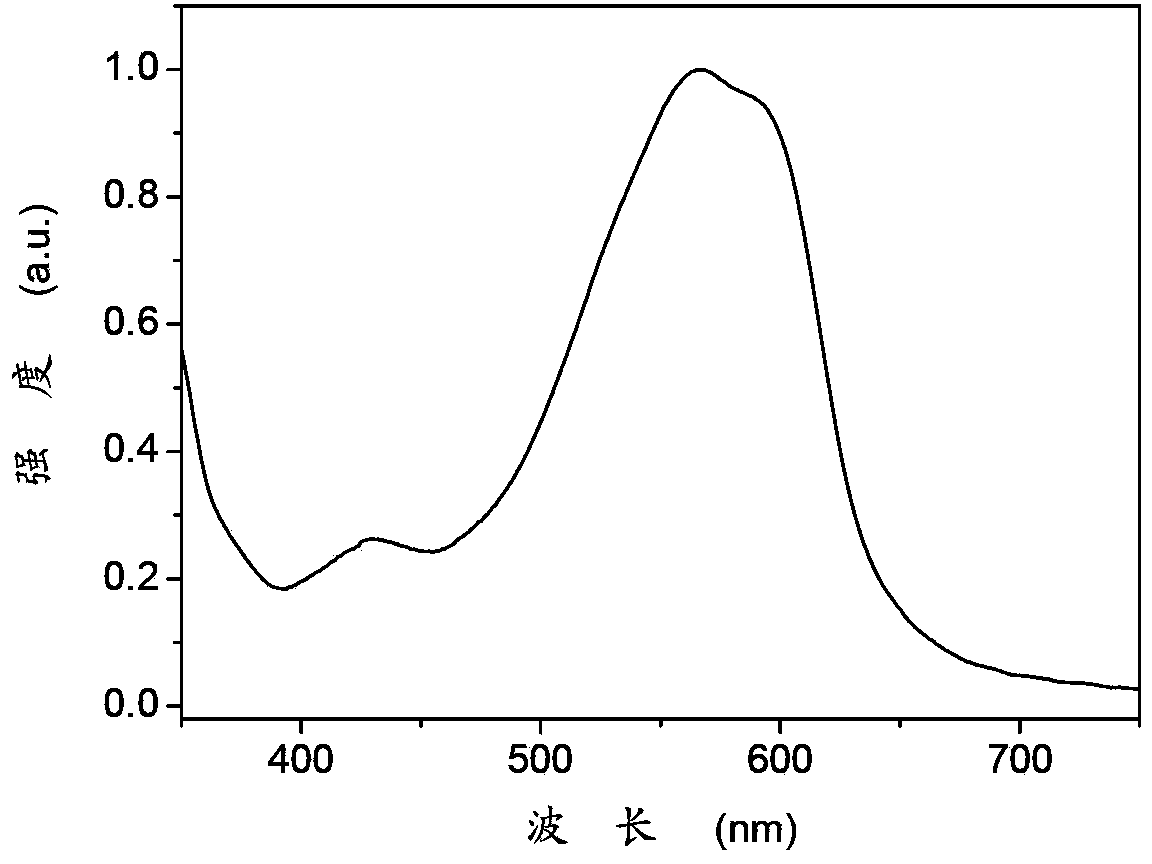
Examples
preparation example Construction
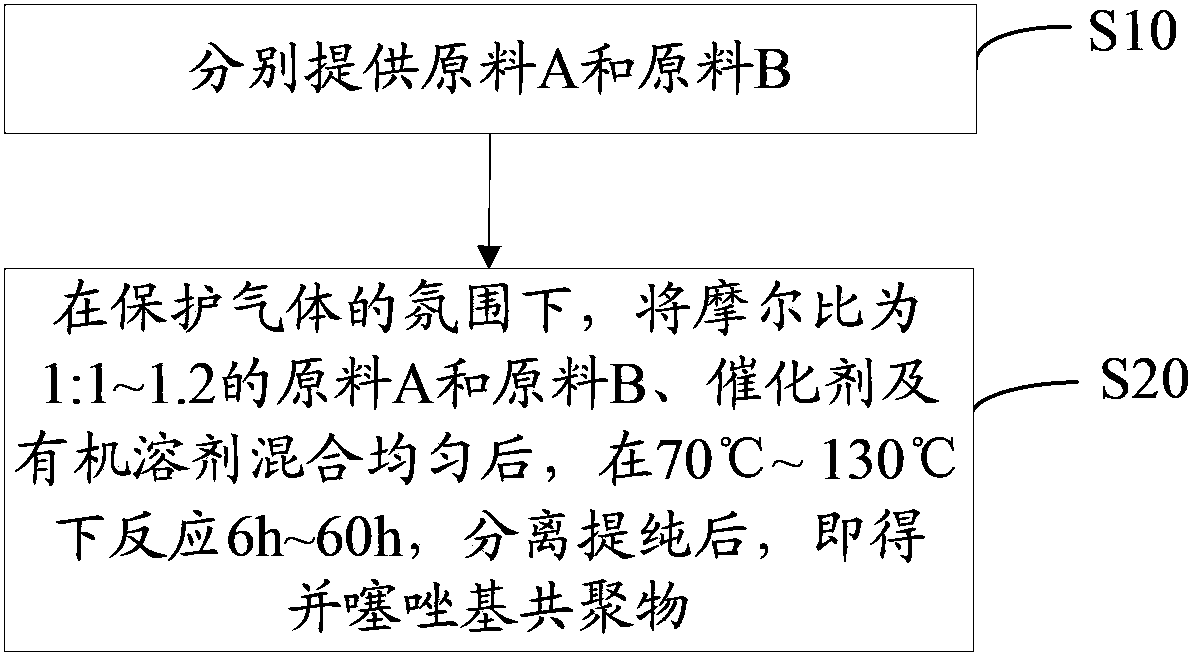
[0033] see figure 1 , the preparation method of the thiazole-based copolymer of one embodiment, comprises the following steps:
[0034] Step S10, providing raw material A and raw material B respectively, the structural formulas of raw materials A and B are as follows: Among them, R 1 , R 2 are selected from C 1 ~C 20 alkyl.
[0035] Both raw material A and raw material B are products produced by Bailingwei Technology Co., Ltd.
[0036] Step S20, under the atmosphere of protective gas, mix raw material A and raw material B, catalyst and organic solvent with a molar ratio of 1:1~1.2 uniformly, react at 70°C~130°C for 6h~60h, separate and purify, That is, the thiazole-based copolymer with the following structural formula is obtained:
[0037]
[0038] Wherein, n is an integer of 10-100.
[0039] Wherein, the reaction temperature is preferably 90°C-120°C, and the reaction time is preferably 12h-48h.
[0040] The protective gas is argon, nitrogen or a mixture of nitrog...
Embodiment 1
[0063] Poly{2,6-diyl-4,8-bis(5-n-octylthiophene)benzodithiophene-co-2,5-bis(5-yl-3-n-octyl-2-thiophene ) Preparation of thiazolo[5,4-d]thiazole} (abbreviated as P1).
[0064] Above-mentioned thiazole-based copolymer P1 is prepared according to the following reaction formula:
[0065]
[0066] 181mg of 2,6-bistrimethyltin-4,8-bis(5-n-octylthiophene)benzodithiophene (0.2mmol), 138mg of 2,5-bis(5-bromo-3- Add n-octyl-2-thiophene)thiazolo[5,4-d]thiazole (0.2mmol) into a flask containing 10mL of toluene solvent, vacuumize and remove oxygen and fill with argon, then add 5.6mg of bistri Phenylphosphinepalladium dichloride (0.008mmol) was subjected to Stille coupling reaction at 100°C for 36h. After the reaction, the solution in the flask was cooled to room temperature, 50mL of methanol was added dropwise to the flask for sedimentation, filtered to obtain a precipitate, and the obtained precipitate was sequentially extracted with methanol and n-hexane for 24 hours by Soxhlet extr...
Embodiment 2
[0070] Poly{2,6-diyl-4,8-bis(5-methylthiophene)benzodithiophene-co-2,5-bis(5-yl-3-n-eicosyl-2-thiophene) Preparation of thiazolo[5,4-d]thiazole} (abbreviated as P2).
[0071] Above-mentioned thiazole-based copolymer P2 is prepared according to the following reaction formula:
[0072]
[0073] 212 mg of 2,6-bistrimethyltin-4,8-bis(5-methylthiophene)benzodithiophene (0.3 mmol), 307 mg of 2,5-bis(5-bromo-3-normal di Add dedecyl-2-thiophene)thiazolo[5,4-d]thiazole (0.3mmol) and 15mL tetrahydrofuran into a 50mL two-neck bottle, fully dissolve and then pass in a mixture of nitrogen and argon to exhaust the air for about 20min Finally, 4mg of tetrakistriphenylphosphine palladium (0.003mmol) was added into the two-necked flask, and the mixed gas of nitrogen and argon was fully ventilated for about 10min, and the Stille coupling reaction was carried out at 70°C for 60h. After the reaction, cool the solution in the two-necked flask to room temperature, add 40 mL of methanol to the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Resistance | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com