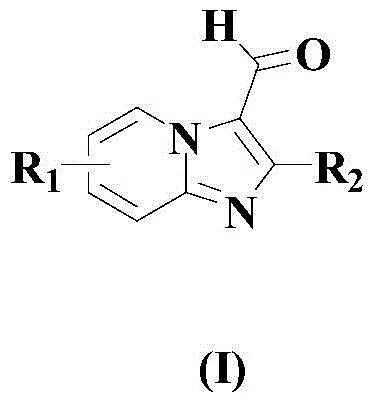
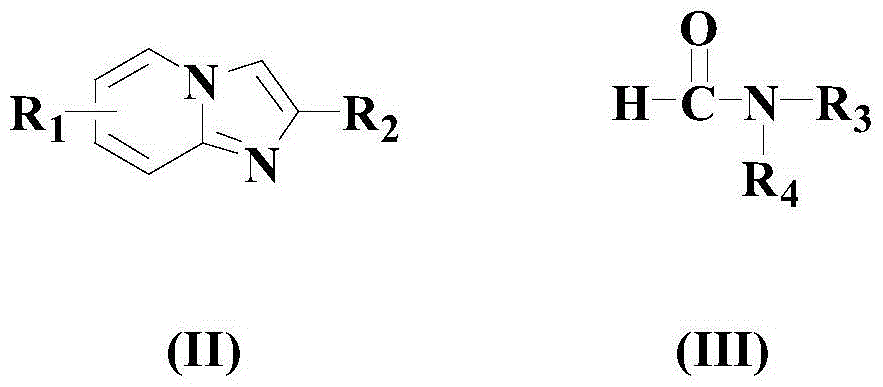
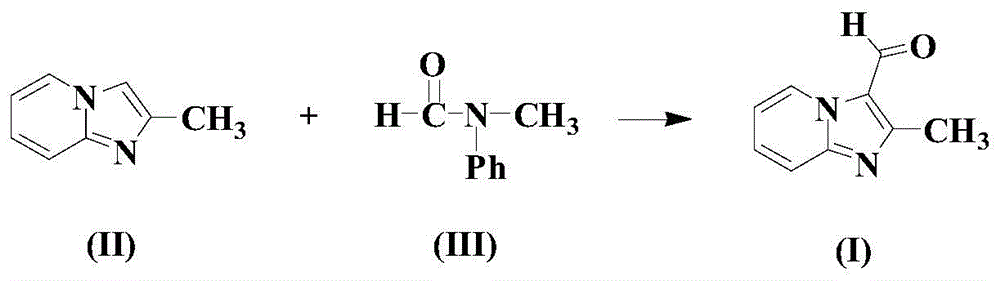A kind of synthetic method of formyl heteroaromatic drug intermediate compound
A technology for the synthesis of formyl heteroaromatic hydrocarbons and synthesis methods, which is applied in the synthesis of heteroaromatic compounds and the synthesis of formyl heteroaromatic drug intermediate compounds. It can solve the problems of few reports on formylation modification methods and achieve excellent technology effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] In a dry reaction vessel, add an appropriate amount of organic solvent toluene, then add 100mmol formula (II) compound, 2mmol copper trifluoroacetylacetonate, 100mmol formula (III) compound, 200mmol TBHP and 5mmol reaction auxiliary (4.2mmol N- A mixture of butylpyridine sulfonate p-toluenesulfonate and 0.8 mmol cyclooctene tricarbonyl iron), the temperature was raised to 70° C., and the reaction was carried out at this temperature for 12 hours.
[0035] After completion of the reaction, cool to room temperature naturally, then add saturated aqueous sodium bicarbonate solution to wash fully, separate the organic phase, dry with anhydrous magnesium sulfate, then concentrate under reduced pressure, and the residue is separated by silica gel column chromatography (wherein the column chromatography is used for washing Dehydration is a mixture of ethyl acetate and n-propanol, the volume ratio of the two is 10:1), and the compound of formula (I) is obtained with a ...
Embodiment 2
[0039]
[0040] In a dry reaction vessel, add an appropriate amount of organic solvent chlorobenzene, then add 100mmol formula (II) compound, 150mmol formula (III) compound, 4mmol copper trifluoroacetylacetonate, 220mmol TBHP and 7mmol reaction auxiliary (6.2mmol N - a mixture of butylpyridine sulfonate p-toluenesulfonate and 0.8 mmol of cyclooctatetraenetricarbonyliron), raise the temperature to 80° C., and react at this temperature for 10 hours.
[0041] After completion of the reaction, cool to room temperature naturally, then add saturated aqueous sodium bicarbonate solution to wash fully, separate the organic phase, dry with anhydrous magnesium sulfate, then concentrate under reduced pressure, and the residue is separated by silica gel column chromatography (wherein the column chromatography is used for washing Dehydration is a mixture of ethyl acetate and n-propanol, the volume ratio of which is 10:2), and the compound of formula (I) is obtained with a yield of 98.6%. ...
Embodiment 3
[0045]
[0046] In a dry reaction vessel, add an appropriate amount of organic solvent N-methylpyrrolidone, then add 100mmol formula (II) compound, 200mmol formula (III) compound, 6mmol copper trifluoroacetylacetonate, 240mmol TBHP and 8mmol reaction auxiliary agent (for 7.25 mmol of N-sulfonic acid butylpyridine p-toluenesulfonate and 0.75 mmol of cyclooctatetraene tricarbonyl iron), the temperature was raised to 100° C., and the reaction was carried out at this temperature for 8 hours.
[0047] After completion of the reaction, cool to room temperature naturally, then add saturated aqueous sodium bicarbonate solution to wash fully, separate the organic phase, dry with anhydrous magnesium sulfate, then concentrate under reduced pressure, and the residue is separated by silica gel column chromatography (wherein the column chromatography is used for washing Dehydration is a mixture of ethyl acetate and n-propanol, the volume ratio of the two is 10:1), and the compound of form...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com