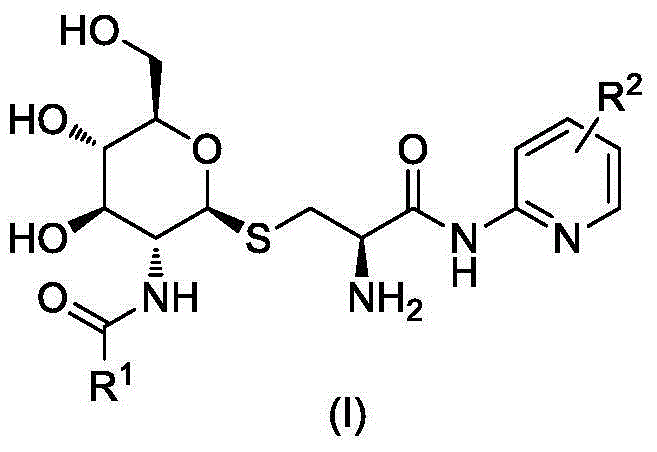
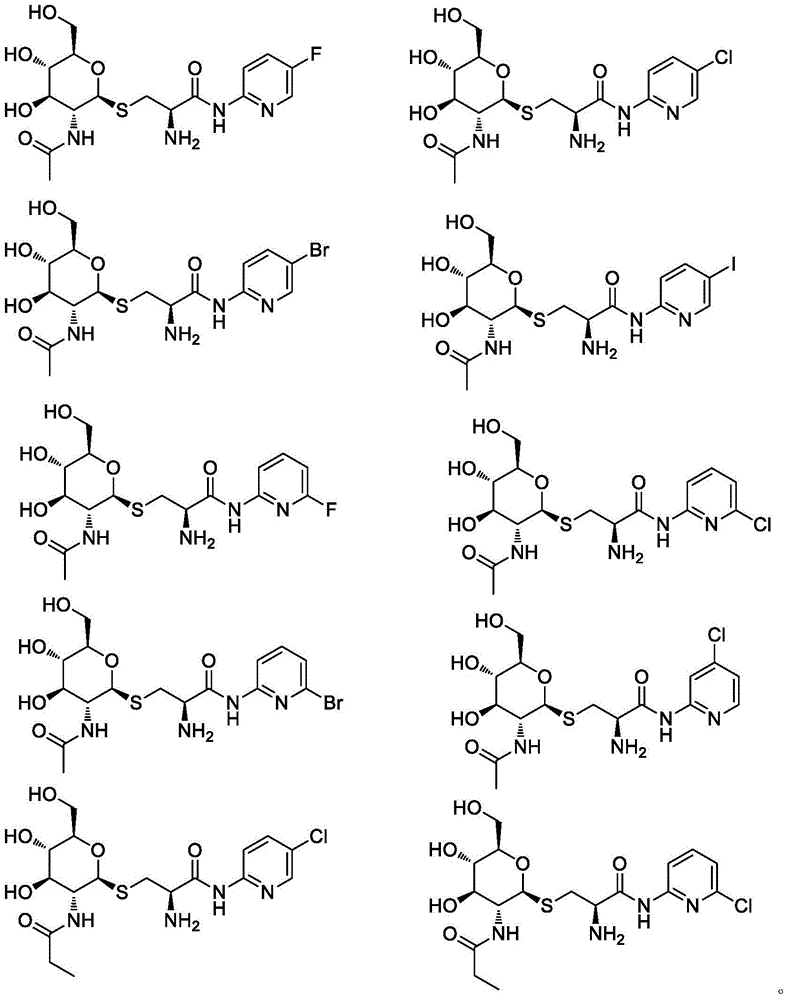
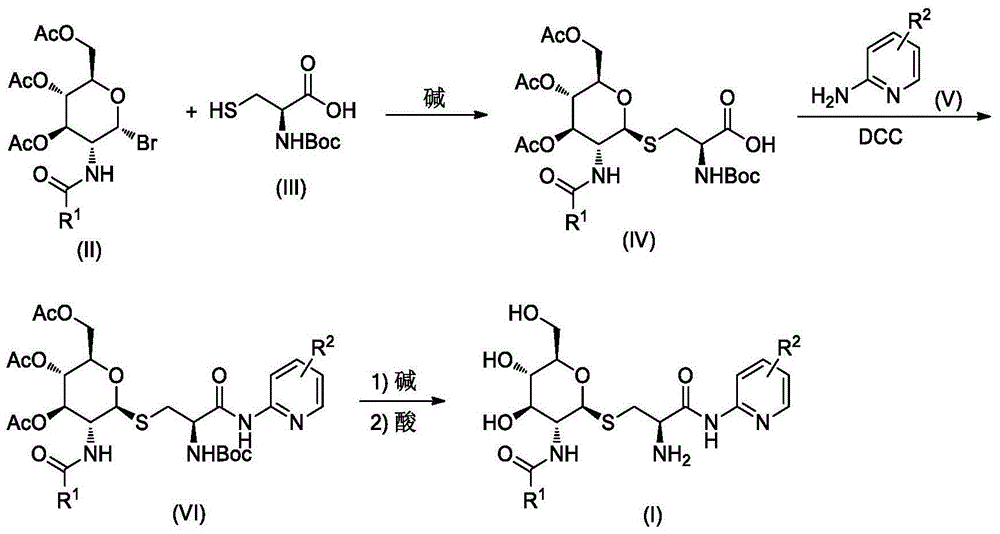
GPR119 agonist containing glucosamine and halogenated pyridine structures and application thereof
An alkyl and halogen technology, applied in the field of GPR119 agonists, can solve the problems of unclear exact cause, loss of sensitivity, and decreased insulin secretion promoting effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The synthesis of embodiment 1 compound I-1
[0030]
[0031] A. Synthesis of Compound IV-1
[0032] Compound II-14.10g (10mmol) and Compound III 2.21g (10mmol) were dissolved in 20mL of dichloromethane, stirred at room temperature, 20mL of 20% NaOH solution and 3g of benzyltriethylammonium bromide were added, and then the reaction mixture was heated at room temperature After stirring overnight, the reaction was checked by TLC for completion. The reaction mixture was poured into 100 mL of ice water, carefully adjusted to pH=2-3 with hydrochloric acid, and 50 mL×3 of CH 2 Cl 2 After extraction, the extract phases were combined, washed with brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the obtained residue was purified by column chromatography to obtain compound IV-1 as a white solid, ESI-MS, m / z=551 ([M+H] + ).
[0033] B. Synthesis of Compound VI-...
Embodiment 2-10
[0038] Referring to the method of Example 1, the compounds listed in the following table were synthesized.
[0039]
[0040]
Embodiment 11
[0041] Preparation of Example 11 Reference Compound D-1
[0042] In order to further illustrate the pharmacological effects, the applicant listed a new compound D-1 (unpublished) and its pharmacological effects researched by the same applicant as a reference.
[0043]
[0044] A. Synthesis of Compound IV-11
[0045] Compound II-11 3.95g (10mmol) and compound III 2.21g (10mmol) were dissolved in 20mL of dichloromethane, stirred at room temperature, 20mL of 20% NaOH solution and 3g of benzyltriethylammonium bromide were added, and then the reaction mixture was heated at room temperature After stirring overnight, the reaction was checked by TLC for completion. The reaction mixture was poured into 100 mL of ice water, carefully adjusted to pH=2-3 with hydrochloric acid, and 50 mL×3 of CH 2 Cl 2 After extraction, the extract phases were combined, washed with brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com