Method for detecting content of moroxydine hydrochloride in Ganmaoqing capsule
A technology of morpholine guanidine hydrochloride and a detection method, which are applied in the content detection of morpholine guanidine hydrochloride and the content detection of active ingredients in pharmaceutical preparations, can solve problems that affect the accuracy of analysis results, the degree of separation is not ideal, and the morpholine hydrochloride is not specified. Guanidine content determination and other problems, to achieve the effect of linearity retention and stability, good quality, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
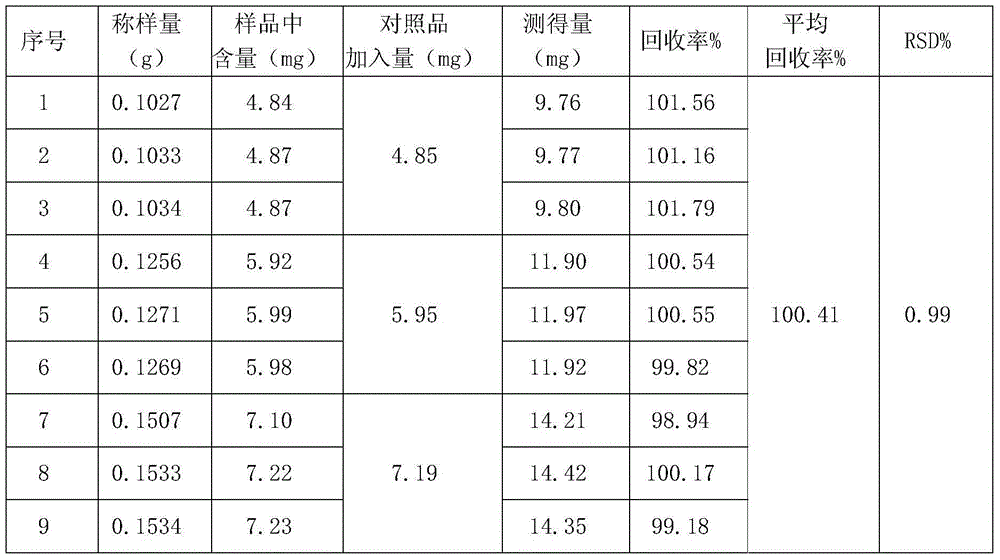
[0011] The detection method of morpholine guanidine hydrochloride content in Ganmaoqing capsules of the present invention is measured with reversed-phase high-performance liquid chromatography, and it is reference substance with morpholine guanidine hydrochloride, with triethylamine (adjust pH to 3.0 with phosphoric acid)-acetonitrile (volume The ratio is 80:20) as the mobile phase. Wherein the high-performance liquid chromatography condition is: with octadecylsilane bonded silica gel as filler, 0.05mol / L triethylamine (adjust pH to 3.0 with phosphoric acid)-acetonitrile as mobile phase with a volume ratio of 80:20, The detection wavelength is 237nm, and the number of theoretical plates is not less than 1200 based on the peak of morpholine guanidine hydrochloride. The specific operation steps are as follows:
[0012] 1) Preparation of reference substance solution: Weigh 12 mg of morpholine guanidine hydrochloride reference substance dried to constant weight at 105°C, put it i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com