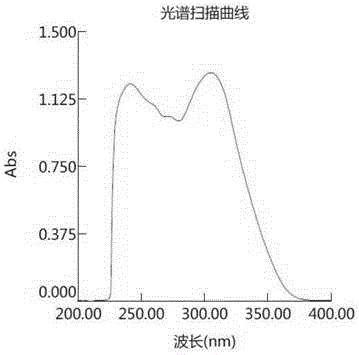
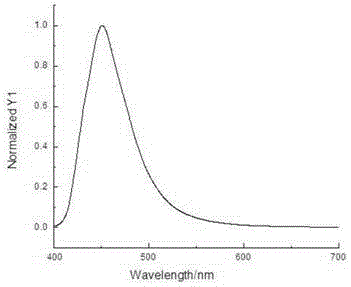
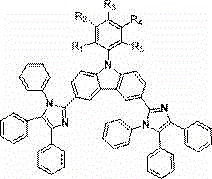
Electron transport materials, preparation method thereof and device comprising electron transport materials
An electron transport material and electron transport technology, applied in the field of organic optoelectronic materials, can solve the problem that carbazole-based light-emitting materials cannot meet the requirements of OLED use, and meet the needs of industrial development, and the synthesis and purification are relatively simple and low-cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
[0037]Load 0.3100mol of iodobenzene, 0.2400mol of carbazole, 0.0218mol of o-phenanthroline, 0.0218mol of cuprous iodide, and 0.3600mol of cesium carbonate into a 1000ml three-necked flask, add 300ml of DMF to dissolve, evacuate - pass nitrogen three times, and heat up to 150°C, react overnight, pass the reaction solution through a silica gel funnel, rinse with dichloromethane until no product is dissolved, spin to about 400ml, wash with 500ml water, separate the liquid, take the dichloromethane phase, wash three times with water, spin dry, add 100ml to dissolve , adding 500 ml of ethanol to precipitate a large amount of solid, suction filtration, and vacuum drying at 55 ° C to obtain 0.2096 mol of N-phenyl-9H-carbazole with a yield of 87.63%. Mass spectrum of the product obtained: theoretical 243.30; found 243.20. Elemental analysis: theoretical value C: 88.86; H: 5.39; N: 5.76; test value C: 88.84; H: 5.40; N: 5.77.
[0038] Load 0.1640mol of N-phenyl-9H-carbazol...
Embodiment 2
[0042]
[0043] 0.2600 mol of p-methyl iodobenzene, 0.2400 mol of carbazole, 0.0218 mol of o-phenanthroline, 0.0218 mol of cuprous iodide, and 0.3600 mol of cesium carbonate were put into a 1000 ml three-necked flask, 300 ml of DMF was added to dissolve, and the vacuum was evacuated and passed through nitrogen three times. , the temperature was raised to 151 °C, and the reaction was carried out overnight. Pass the reaction solution through a silica gel funnel, rinse with dichloromethane until no product is dissolved, spin to about 400ml, wash with 500ml of water, separate the liquid, take the dichloromethane phase, and wash with water three times; The solid was precipitated, filtered with suction, and dried under vacuum at 55°C. 0.2088 mol of 9-p-methylphenyl-9H-carbazole was obtained with a yield of 87.00%. Mass spectrum of the product obtained: theoretical 257.33; found 257.30. Elemental analysis: theoretical value C: 88.68; H: 5.88; N: 5.44; test value C: 88.66; H: 5.8...
Embodiment 3
[0048]
[0049] Put 0.288mol of 3,4-dimethyliodobenzene, 0.24mol of carbazole, 0.02mol of o-phenanthroline, 0.02mol of cuprous iodide, and 0.384mol of cesium carbonate into a 1000ml three-necked flask, add 300ml of DMF to dissolve, and vacuumize - Pass nitrogen three times, heat up to 152°C, and react overnight. Pass the reaction solution through a silica gel funnel, rinse with dichloromethane until no product is dissolved; rotate to about 400ml, wash with 500ml water, separate the liquid, take the dichloromethane phase, and wash with water three times; spin dry, add 100ml to dissolve, add 500ml ethanol, there will be a lot of The solid was precipitated, filtered with suction, and dried under vacuum at 55°C. 0.2136 mol of 9-(3,4-dimethylbenzene)-9H-carbazole was obtained with a yield of 89.00%. Mass spectrum of the product obtained: theoretical 271.36; found 271.34. Elemental analysis: theoretical value C: 88.52; H: 6.31; N: 5.16; test value C: 88.50; H: 6.32; N: 5.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com