Antibody drug conjugate with alkenylsulphonylamino as connexon and application of antibody drug conjugate
An alkenyl sulfonamide-based, antibody drug technology is applied in the directions of drug combinations, anti-tumor drugs, medical preparations with non-active ingredients, etc., to achieve high stability and improved anti-tumor activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Because colchicine is too toxic, it cannot be used to treat cancer. In this example, the activity of colchicine derivatives linked with alkenyl sulfonamide groups was tested and linked to herceptin drug molecules, thereby preparing a new antibody-drug conjugate.
[0089] (1) Preparation of Colchicine Derivative 9
[0090] This example provides a method for preparing an antibody-drug conjugate with an alkenylsulfonamide group as a linker. First, a Boc group is attached to the amide bond of colchicine to obtain compound 5, and then the acetyl group is selectively removed with sodium methoxide to obtain compound 6, and finally the Boc group is removed with trifluoroacetic acid, so that the deacetylated compound is obtained. Compound 7, the primary amine in this structure was reacted with 2-chloroethanesulfonyl chloride to obtain colchicine derivative 9 containing alkenylsulfonamide linker.
[0091] The synthetic route is as follows:
[0092]
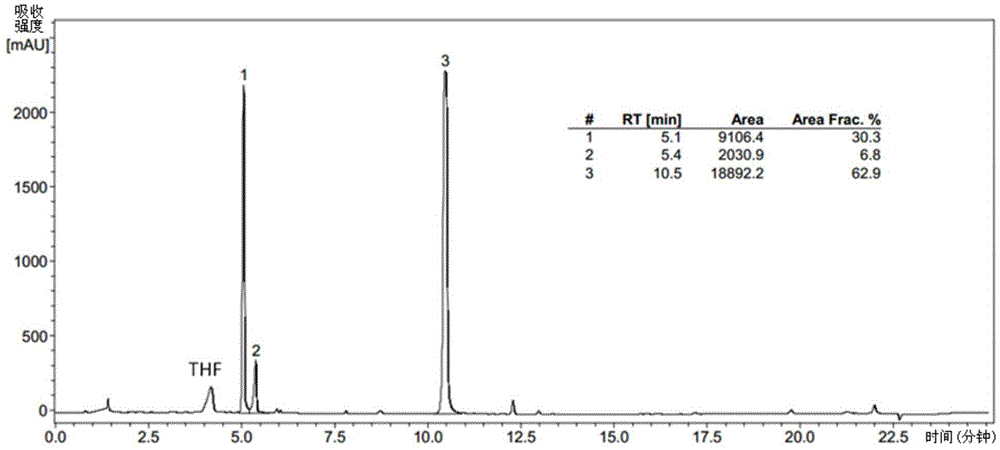
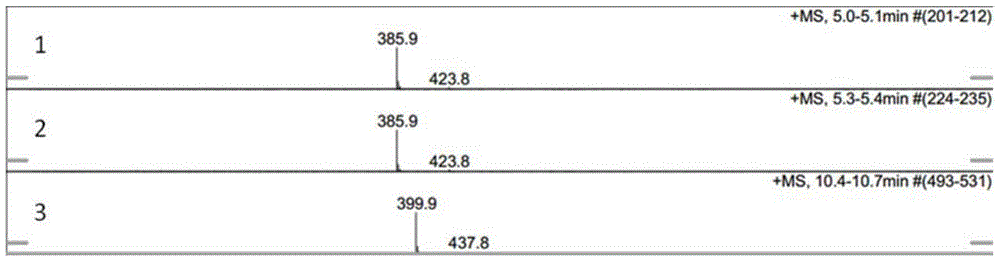
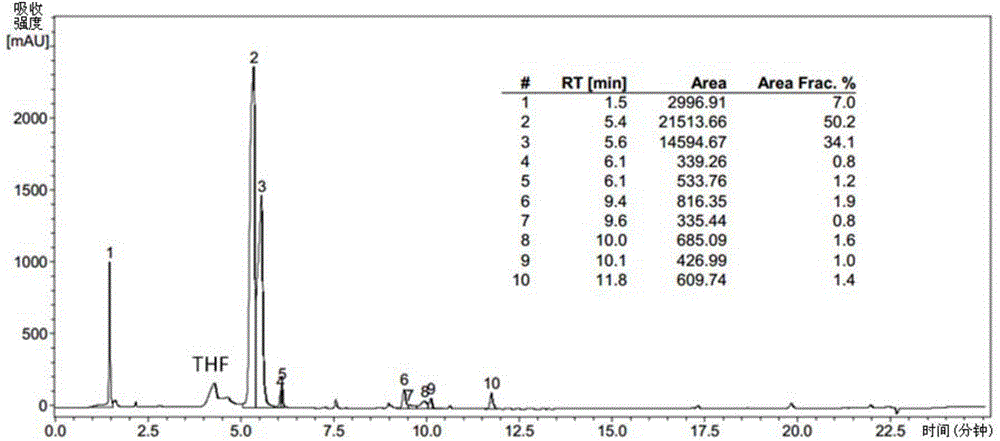
[0093] The specific pre...
Embodiment 2
[0101] Embodiment 2: ELISA test of colchicine-Herceptin antibody conjugate
[0102] 100uL of 0.05μg / mL human HER2 protein (coating buffer is 0.05M carbonate buffer solution, pH=9.6) was added to the microwells and incubated overnight at 4°C, and washed with 3% skimmed milk in PBS. Add 100uL of Trastuzumab and Trastuzumab-colchicine conjugates of various concentrations to each well and incubate at room temperature for 1 hour, then add HRP-conjugated goat anti-human IgG (1:10,000) and incubate for 1 hour, add 50uL TMB substrate into each well, with an equal volume of H 2 SO 4 Stop the reaction and read the absorbance at 450nm.
[0103] join Figure 19 , the figure shows the ELISA test chart of the binding of Herceptin antibody and colchicine-Herceptin antibody conjugate to the antigen respectively, Her in the figure means Herceptin antibody, and Her-col means colchicine-Herceptin antibody Conjugates. Figure 19 The results of the data show that the antigen-binding ability o...
Embodiment 4
[0104] Example 4: Test of colchicine-Herceptin antibody conjugate against cancer cell SKBR3
[0105] After the cancer cell SKBR3 was inoculated in a 96-well plate and adhered to the wall overnight, the supernatant was discarded, and fresh medium containing different concentrations of antibodies was added. After 72 hours of treatment, MTS reagent (Promega Company) was added to continue incubation for 2 hours, and the absorbance at 490 nm was read with a microplate microplate reader. see Figure 20 , the figure is a schematic diagram of the inhibitory activity of Herceptin antibody and colchicine-Herceptin antibody conjugate on SKBR3 cells, where Herceptin means Herceptin antibody, and Herceptin-Colchincine means colchicine-Herceptin antibody conjugate things. Figure 20 , the abscissa indicates the concentration of Herceptin antibody and colchicine-Herceptin antibody conjugate, and the ordinate indicates the relative activity of SKBR3 cells. From Figure 20 It can be seen t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com