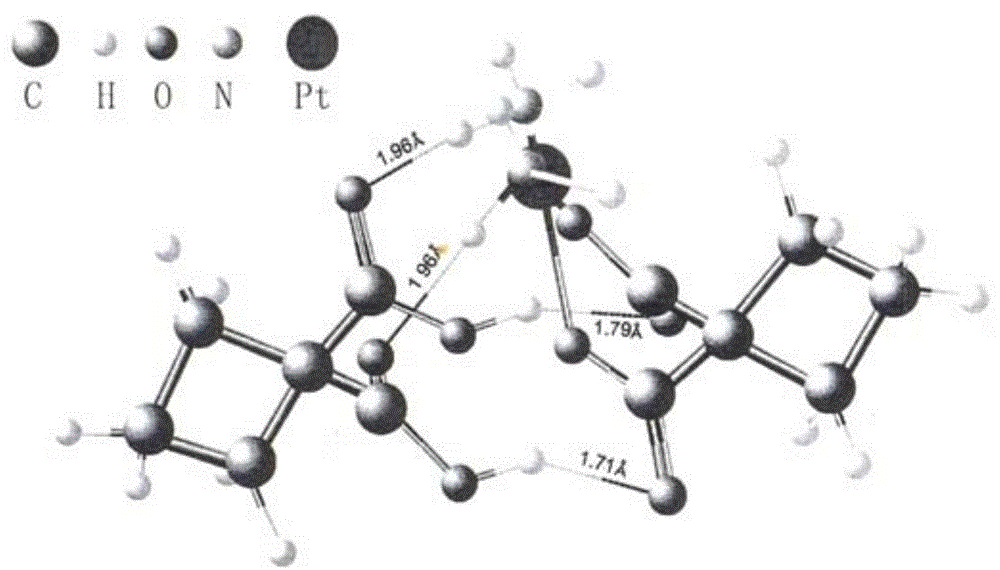
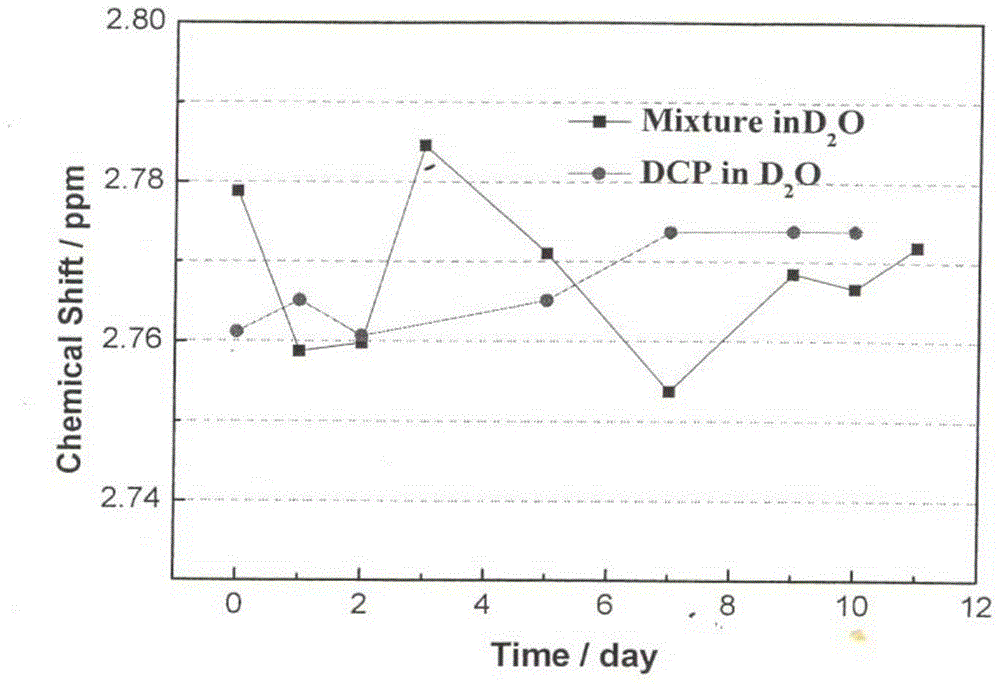
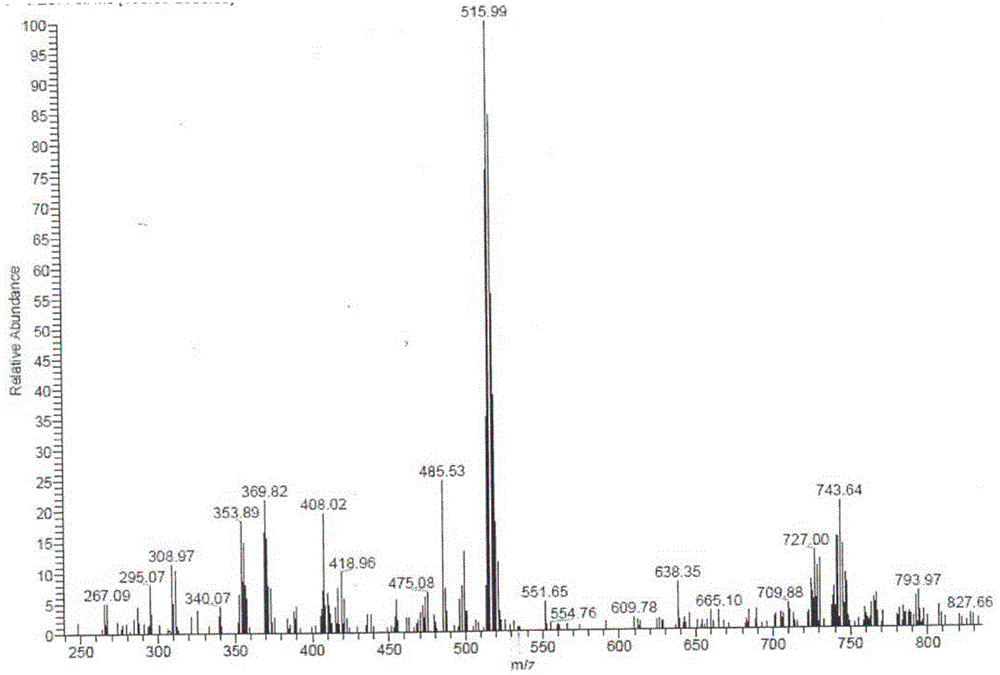
Preparation method of supramolecular anti-cancer drug (dicycloplatin)
An anticancer drug, bicycloplatinum technology, applied in the field of preparation of supramolecular anticancer drug bicycloplatinum, can solve the problems of easy destruction of hydrogen bonds, inappropriate distilled water post-treatment steps, low yield and the like, and achieves simplified determination process and conditions. Gentle, high-yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Add 37.1g (0.1mol) of carboplatin and 14.4g (0.1mol) of 1,1-cyclobutanedicarboxylic acid into 1855mL (50 times the amount) of water for injection, and stir at room temperature (about 25°C) for about 1 hour to The solids were all dissolved, and then left to stand in the dark at room temperature for 7 days; concentrated under reduced pressure at 40°C, and dried to obtain 51.5 g of bicycloplatinum qualified for inspection, with an HPLC purity of 99.95% and a yield of 100% ( 1 H-NMR see Figure 4 , see ESI Figure 5 , XRPD spectrum see Image 6 , the XRPD spectrum of carboplatin is shown in Figure 7 ).
[0043] Figure 4 bicycloplatin in 1 H-NMR analysis is as follows:
[0044] 1.816-1.736ppm, 5-fold peak, coupling constant J=8Hz, 2 H, which is the 3-position CH on the left four-membered ring in structural formula (I) 2 of 2 H atoms.
[0045] 1.928-1.848ppm, 5-fold peak, coupling constant J=8Hz, 2 H, is the 3-position CH of the right four-membered ring in structura...
Embodiment 2
[0058] Add 37.1g (0.1mol) of carboplatin and 14.4g (0.1mol) of 1,1-cyclobutanedicarboxylic acid into 1855mL (50 times the amount) of water for injection, and stir at about 15°C for about 2 hours until all the solids are Dissolved, and then stood in the dark at 15° C. for 9 days; concentrated under reduced pressure at 50° C., dried to obtain 50.5 g of fully qualified bicycloplatinum, HPLC purity 99.90%, and yield 98% (see ESI spectrogram Figure 8 ).
Embodiment 3
[0060] Add 37.1g (0.1mol) of carboplatin and 14.4g (0.1mol) of 1,1-cyclobutanedicarboxylic acid into 1855mL (50 times the amount) of water for injection, and stir at about 40°C for about 1 hour until all the solids are Dissolved, and then stood in the dark at 40°C for 6 days; concentrated under reduced pressure at 30°C, and dried to obtain 51.5g of fully qualified bicycloplatinum, with an HPLC purity of 99.96% and a yield of 100% (see ESI spectrogram Figure 9 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com