Chloromethyl methyl ether and synthesis method of compound protected by methoxymethyl thereof
A technology of chloromethyl methyl ether and synthesis method, applied in the direction of preparation of organic compounds, steroids, chemical instruments and methods, etc., can solve the problem of easy hydrolysis deactivation, blockage of reactor pipes, and unsuitability for continuous flow reactors and other issues, to achieve the effect of fast response speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
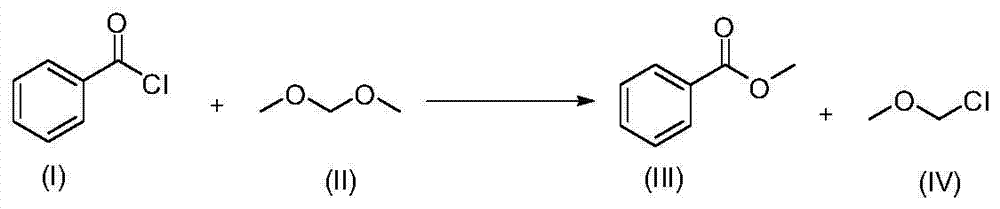
[0025] The synthesis of embodiment 1 chloromethyl methyl ether (MOMCl) (IV)
[0026]
[0027] Dissolve 26mL (0.225 mol) of benzoyl chloride (I) and 20mL (0.225 mol) of dimethoxymethane (II) in a 100mL reaction flask, add 0.6mL (3 mol%) of trifluoromethanesulfonic acid, and next reaction, 1 H NMR monitoring, 0.5h reaction complete. Post-processing: the resulting reaction solution was pumped into a cleaned cold trap under a water bath, and then distilled at 80°C under normal pressure to obtain 17.85 g of pure product MOMCl(IV), with an isolation yield of 98%.
[0028] Compound (IV): molecular weight, 80.51; 1 H NMR (400MHz, CDCl 3 ):δ5.45(s,2H),3.50(s,3H).
[0029](NMR references: Reggelin, M.; Doerr, S.A Modified Low-cost Preparation of Chloromethyl Methyl Ether (MOM-Cl). Synlett. 2004, 6, 1117-1117.)
Embodiment 2
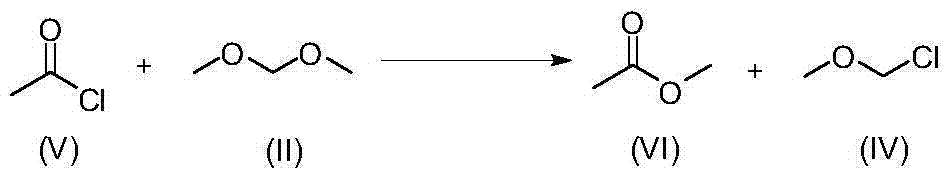
[0030] Embodiment 2 The synthesis of chloromethyl methyl ether (MOMCl) (IV)
[0031]
[0032] Dissolve 16mL (0.225 mol) of acetyl chloride (V) and 20mL (0.225 mol) of dimethoxymethane (II) in a 100mL reaction flask, and slowly add 0.6mL (3 mol%) of trifluoromethanesulfonate at 0°C acid, and then raised to room temperature at 20°C to react, 1 H NMR monitoring, the reaction is complete in less than 10 minutes.
[0033] Compound (IV): molecular weight, 80.51; 1 H NMR (400MHz, CDCl 3 ):δ5.45(s,2H),3.50(s,3H).
[0034] Compound (VI): molecular weight, 74.08; 1 H NMR (400MHz, CDCl 3 ): δ3.66(s,3H),2.05(s,3H).
Embodiment 3
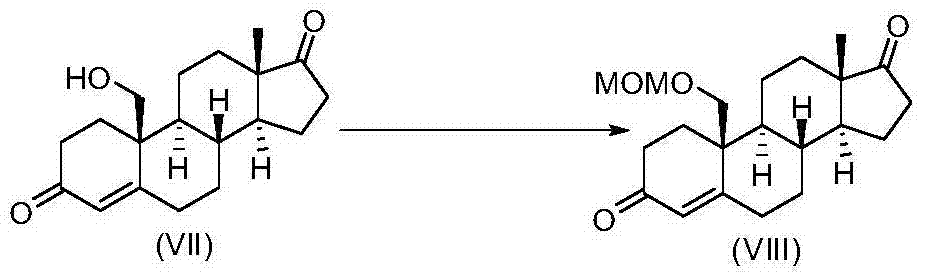
[0035] Example 3 Synthesis of 19-methoxymethoxyandrost-4-ene-3,17-dione (VIII)
[0036]
[0037] Dissolve 5.2 mL (0.045 mol) of benzoyl chloride and 4 mL (0.045 mol) of dimethoxymethane in a 25 mL reaction flask, add 0.12 mL (3 mol %) of trifluoromethanesulfonic acid, and react at 60°C for 0.5 h, After cooling to room temperature, directly and slowly add 6.80g (0.0225 moles) of 19-hydroxyandrost-4-ene-3,17-dione (VII) and 14.9mL (0.09 moles) of N,N-diisopropyl 22.5 mL of dichloromethane solution of ethyl ethylamine to react for 0.5 hour, or monitor the reaction by thin layer chromatography (TLC) until complete. Post-treatment: Pour the reaction system into 30 mL of saturated sodium bicarbonate solution, add 10 mL of dichloromethane, stir thoroughly for 10 minutes, separate the liquids, extract the aqueous phase twice with 15 mL of dichloromethane each time, combine the organic phases, and then wash with water , washed with saturated brine, dried over anhydrous sodium sulfa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com