A kind of glycoprotein enrichment and purification method
A purification method and protein technology, which are applied in chemical instruments and methods, peptide preparation methods, organic chemistry, etc., can solve the problems of large loss of enriched glycoprotein samples, inability to replace solvents, and complicated steps, so as to reduce the loss of samples. , Easy to operate, efficient enrichment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
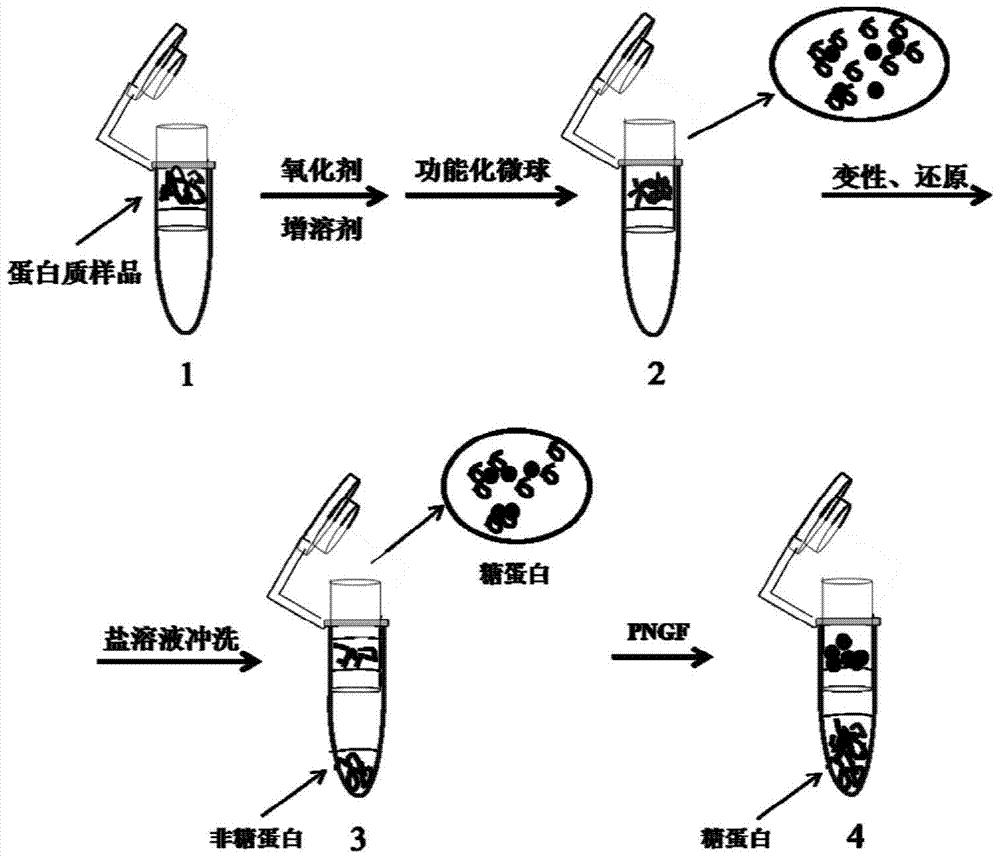
[0022] 1. Oxidation of Glycoproteins
[0023] The whole protein sample extracted from rat liver was dissolved in 10mM sodium periodate solution prepared by 4% SDS oxidation buffer (100mM sodium acetate, 150mM sodium chloride, pH5.5), and the sample solution was added to the ultrafiltration React for 1 h in the ultrafiltration tube of a centrifugal ultrafiltration device with a membrane pore size of 0.45um (purchased from Pall Corporation, Port Washington, USA);
[0024] 2. Removal of excess oxidants
[0025] After the oxidation reaction is over, add oxidation buffer solution to the ultrafiltration tube to prepare a 20 mM sulfite solution for 10 minutes to neutralize excess oxidant;
[0026] 3. Capture of Glycoproteins
[0027] Add agarose gel with hydrazide groups suspended in the oxidation buffer to the ultrafiltration tube, and shake at room temperature for 12 hours;
[0028] 4. Protein denaturation and reduction
[0029] The sample processed in step 3 was centrifuged at...
Embodiment 2
[0047] The whole protein sample extracted from rat liver in Example 1 was replaced with the whole protein sample extracted from human cervical cancer cells; the enrichment steps of the glycoprotein sample were the same as in Example 1.
[0048] Identification result
[0049] total protein number identified
Embodiment 3
[0051] The steps of the oxidation method of glycoproteins are as follows: the 10mM sodium periodate solution prepared with 1% Triton X-100 oxidation buffer solution (100mM sodium acetate, 150mM sodium chloride, pH5.5) is used to dissolve the glycoprotein extracted from rat liver. Whole protein sample; glycoprotein sample enrichment steps are the same as in Example 1.
[0052] Identification result
[0053] total protein number identified
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com