Cu-TiO2 photocatalyst and preparation method thereof
A photocatalyst, titanium dioxide technology, applied in the direction of chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc., can solve the problem of slow degradation of pollutants, time-consuming, discontinuous and other problems, to achieve the effect of simplifying the preparation process, saving preparation time, and improving the degradation speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0012] A Cu-TiO 2 The preparation method of photocatalyst is specifically carried out according to the following steps:
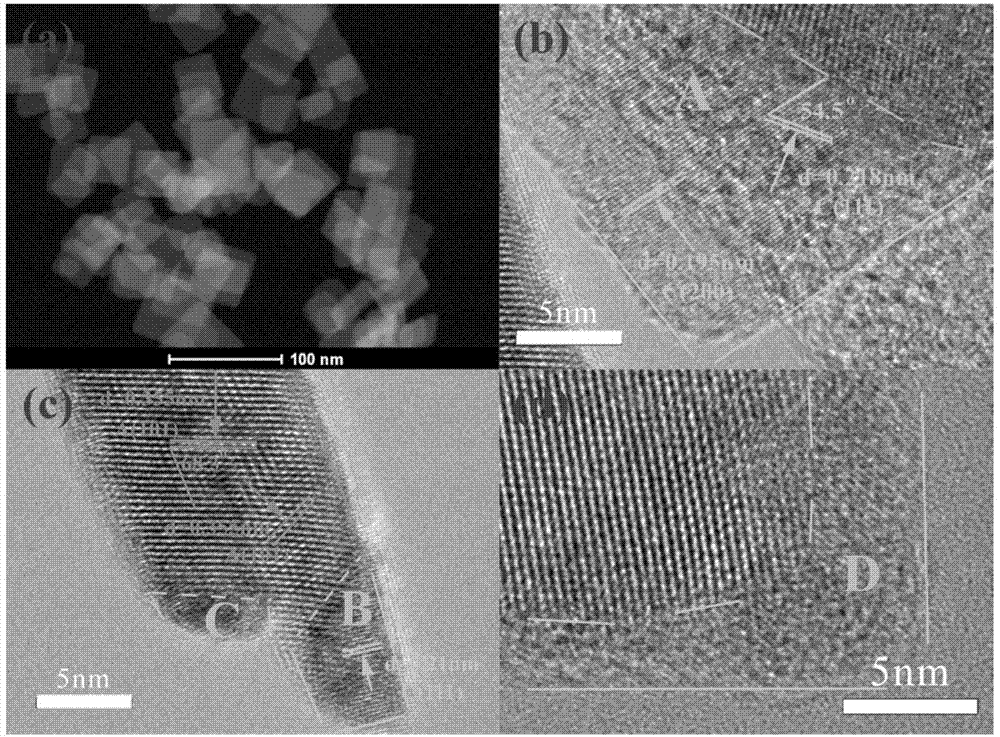
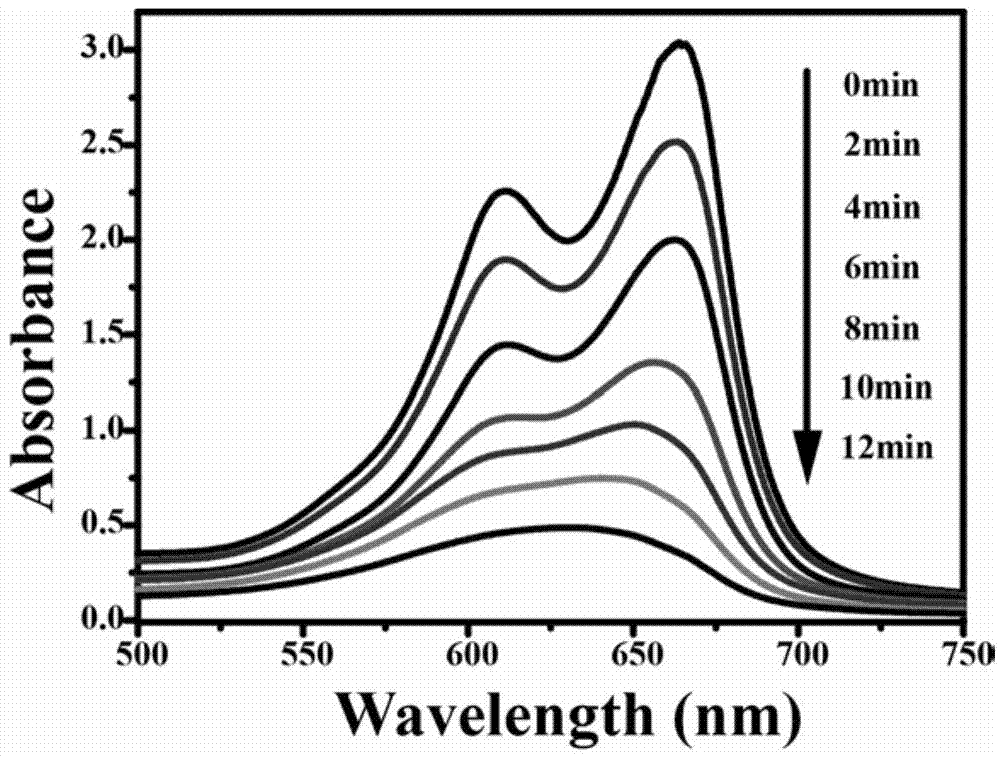
[0013] Add 2.5ml-3.5ml of hydrofluoric acid and 0.6g-0.8g of copper nitrate to 28ml-32ml of tetrabutyl titanate, stir for 12 hours to form a blue solution, and react with hydrothermal synthesis at 175°C-185°C for 12 hours. After the reaction, filter, wash, dry and grind to obtain Cu-TiO 2 catalyst of light.
Embodiment 1
[0015] Add 3ml of hydrofluoric acid and 0.7g of copper nitrate to 30ml of n-butyl titanate, stir for 12 hours to form a blue solution, and conduct a hydrothermal synthesis reaction at 180°C for 12 hours. After the reaction, suction filter, wash, dry and grind , namely to get Cu-TiO 2 catalyst of light.
Embodiment 2
[0017] Add 2.5ml of hydrofluoric acid and 0.6g of copper nitrate to 28ml of n-butyl titanate, stir for 12 hours to form a blue solution, and conduct a hydrothermal synthesis reaction at 175°C for 12 hours. After the reaction, suction filter, wash, dry and Grinding to get Cu-TiO 2 catalyst of light.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com