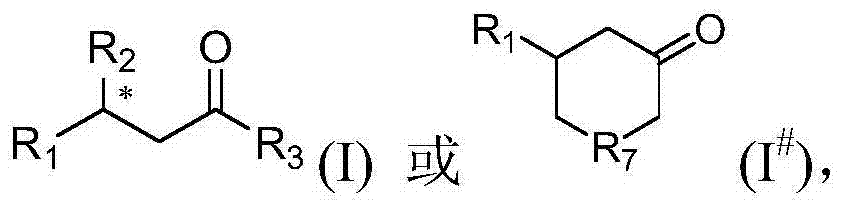
Method for preparing optically active aldehyde or ketone and preparation method of catalyst thereof
An optically active and catalyst technology, which is applied in the preparation of organic compounds, catalysts for physical/chemical processes, and organic chemistry methods, can solve problems such as low hydrogenation efficiency, reduced catalyst conversion frequency, and short catalyst life.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
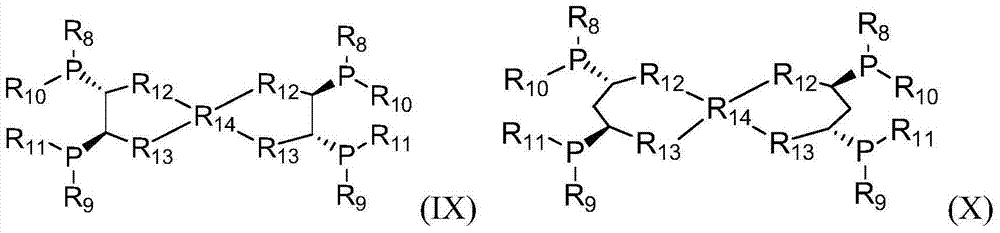
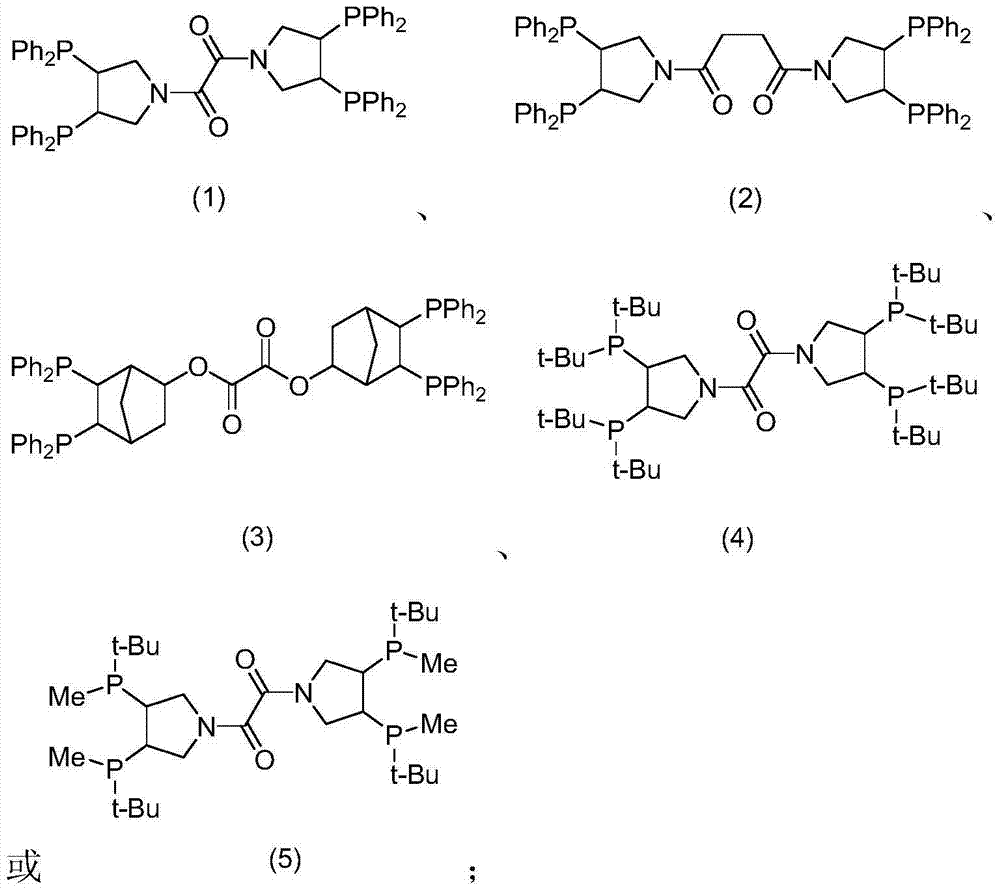
[0078] Synthesis of Oxalo[(3R,4R)-3,4-Bis(diphenylphosphino)pyrrolidine]diamine
[0079] a) Synthesis of (3S,4S)-1-benzyl-3,4-bis(methylsulfonyloxy)pyrrolidine
[0080] Dissolve 16.0g (3S,4S)-(+)-1-benzyl-3,4-pyrrolidinediol and 23.1ml triethylamine in 50ml dichloromethane, add 12.9ml methanesulfonate dropwise at 0°C Acyl chloride, heated up to room temperature and reacted for 30 minutes, then extracted twice with 30ml deionized water, the organic phase was washed with 300ml of 1mol / L hydrochloric acid solution, and the water phase was separated, and the water phase was washed with 100ml of caustic soda solution, then 60ml of dichloromethane was added, and the liquid was separated Finally, the organic phase was kept, and the solvent was removed under reduced pressure to obtain 25.2 g of solid (3S,4S)-1-benzyl-3,4-bis(methylsulfonyloxy)pyrrolidine.
[0081] b) Synthesis of (3S,4S)-3,4-bis(methylsulfonyloxy)pyrrolidine acetate
[0082] Dissolve 24.0g (3S,4S)-1-benzyl-3,4-bis(m...
Embodiment 2
[0088] Synthesis of oxalo[(3R,4R)-3,4-bis(di-tert-butylphosphino)pyrrolidine]diamine
[0089] a) Synthesis of (3R,4R)-3,4-bis(di-tert-butyl)pyrrolidine hydrochloride
[0090] Dissolve 36.2ml of di-tert-butylphosphine in 50ml of dioxane, add 4.67g of sodium metal, stir for 14 hours, then dissolve the reactant in 170ml of N,N-dimethylformamide, cool to -40°C 15.97g (3S,4S)-3,4-bis(methylsulfonyloxy)pyrrolidine acetate was added to the reaction liquid, and the temperature was raised to room temperature for 24 hours. After the reaction was completed, the solvent was removed under reduced pressure, 200ml of 2mol / L hydrochloric acid solution was added, stirred for 1 hour and then filtered. The filter cake was washed with 100ml of deionized water and 200ml of ether and dried to obtain 16.8g of solid, namely (3R,4R)-3, 4-bis(di-tert-butyl)pyrrolidine hydrochloride.
[0091] b) Synthesis of oxalo[(3R,4R)-3,4-bis(di-tert-butyl)pyrrolidine]diamine
[0092] Dissolve 0.879g (3R,4R)-3,4-...
Embodiment 3
[0094] 100.8mg Rh(CO) 2 acac (about 0.4 mmol) and 277.2 mg of oxalo[(3R,4R)-3,4-bis(diphenylphosphino)pyrrolidine]diamine ligand (about 0.3 mmol) were dissolved in 30 g of toluene under argon atmosphere , stirred at room temperature and normal pressure for 5 hours, and after the solution was clarified, a homogeneous catalyst solution was obtained, and the solvent was removed by distillation under reduced pressure at room temperature (vacuum degree <200Pa) to obtain 378.8 mg of a solid catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical purity | aaaaa | aaaaa |
| Optical purity | aaaaa | aaaaa |
| Optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com