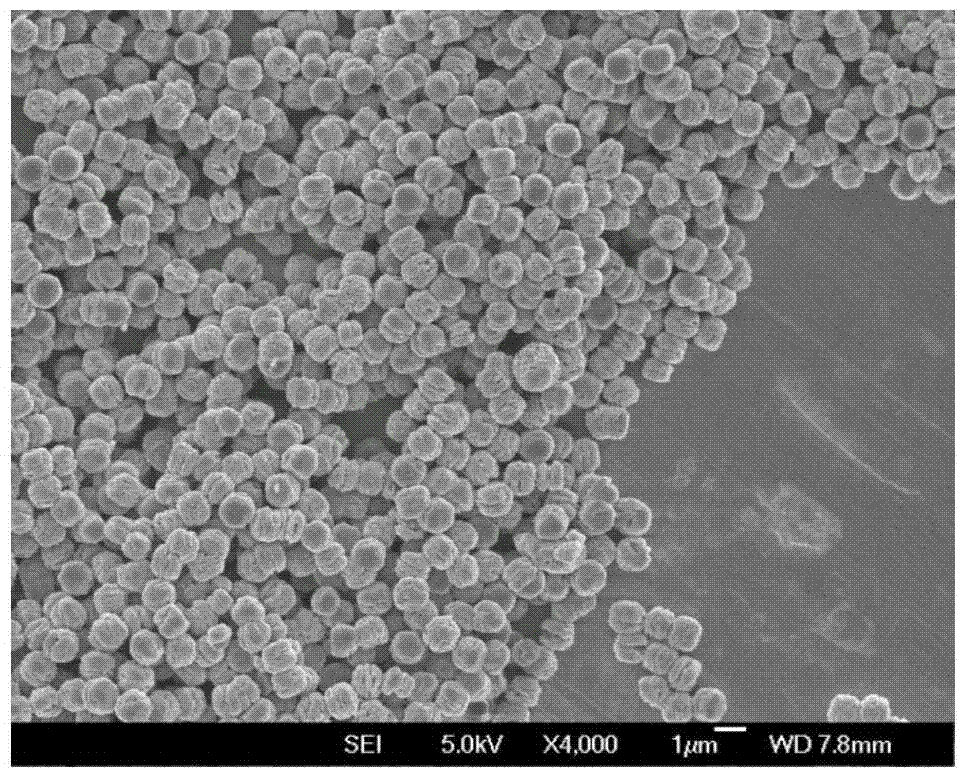
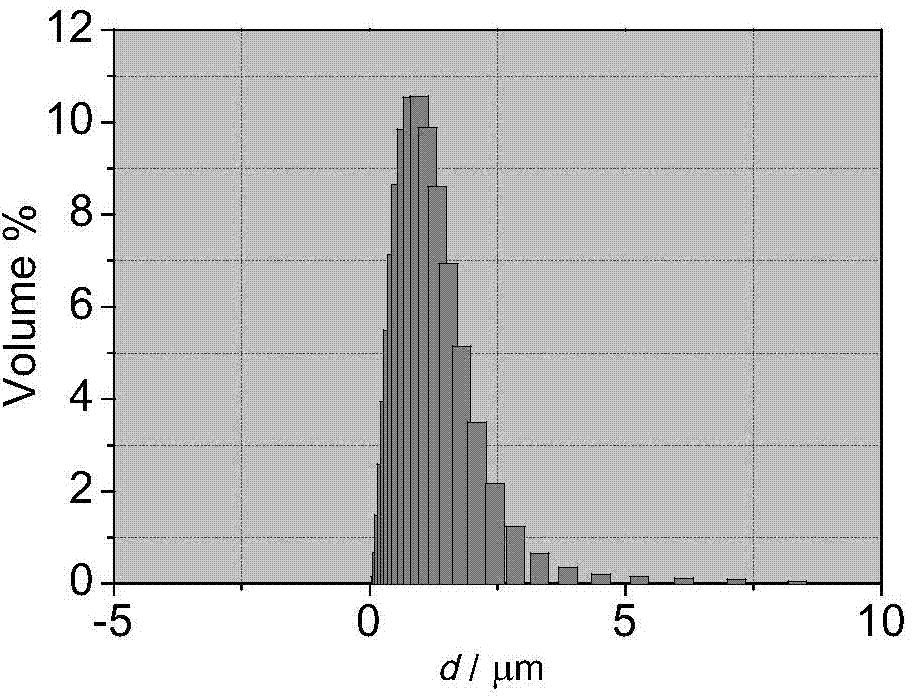
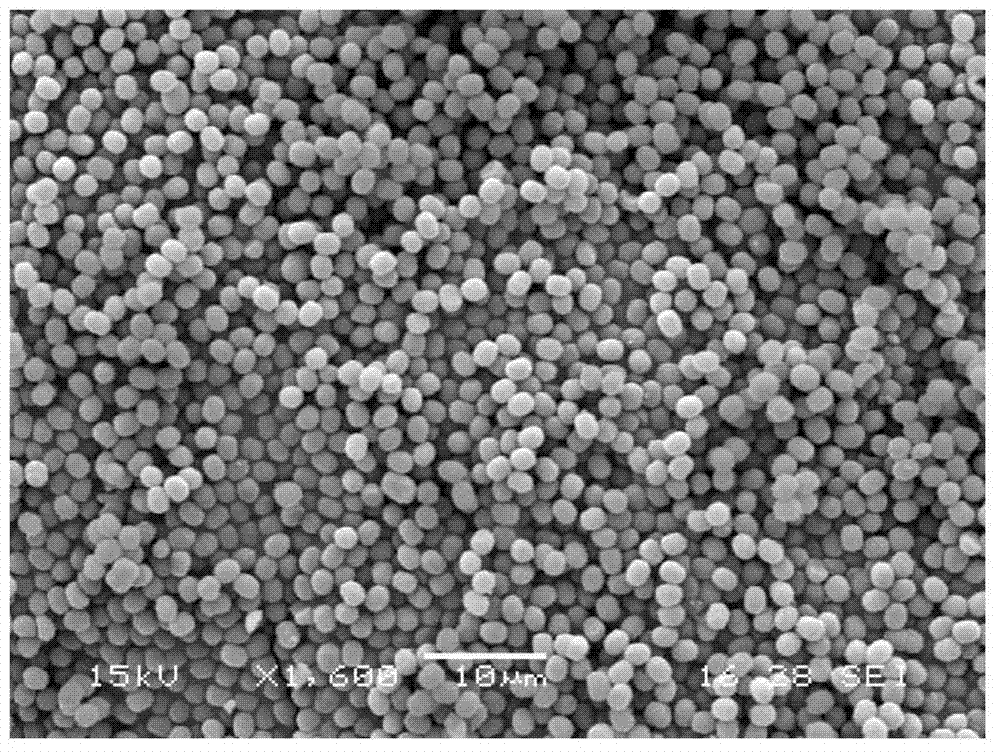
Preparation method of mono-dispersed colloid particles
A colloidal particle, monodisperse technology, applied in the direction of microsphere preparation, microcapsule preparation, etc., to achieve the effect of adjustable chemical composition of particles, uniform and controllable particle size distribution, and adjustable shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] A certain amount of Al 2 (SO 4 ) 3 18H 2 O dissolved in deionized water to form a clear and transparent solution. This solution was named solution one. Then a certain amount of anhydrous sodium acetate (NaAC) was dissolved in deionized water to form a clear and transparent solution. This solution was named solution two. Solution two was added to solution one to form a clear and transparent solution, and the mixed solution was stirred at room temperature for 1 hour to fully mix the two solutions. The final mixture composition molar ratio is Al 2 (SO 4 ) 3 18H 2 O:NaAC:H 2 O=1:4:247. The mixture was transferred to a stainless steel crystallization autoclave lined with polytetrafluoroethylene, and the crystallization autoclave was placed in an oven at 200 °C for 24 hours for hydrothermal treatment. After the hydrothermal treatment was completed, the solid product in the crystallization tank was centrifuged and washed three times with deionized water, and then t...
Embodiment 2
[0038] A certain amount of KAl(SO 4 ) 2 12H 2 O dissolved in deionized water to form a clear and transparent solution. This solution was named solution one. Then a certain amount of anhydrous sodium acetate was dissolved in deionized water to form a clear and transparent solution. This solution was named solution two. Solution two was added to solution one to form a clear and transparent solution, and the mixed solution was stirred at room temperature for 1 hour to fully mix the two solutions. The final mixture composition molar ratio is KAl(SO 4 ) 2 12H 2 O:NaAC:H 2 O=1:2:176. The mixture was transferred to a stainless steel crystallization autoclave lined with polytetrafluoroethylene, and the crystallization autoclave was placed in an oven at 200 °C for 24 hours for hydrothermal treatment. After the hydrothermal treatment was completed, the solid product in the crystallization tank was centrifuged and washed three times with deionized water, and then the solid was ...
Embodiment 3
[0041] A certain amount of Al(NO 3 ) 3 9H 2O dissolved in deionized water to form a clear and transparent solution. This solution was named solution one. Then a certain amount of anhydrous sodium acetate was dissolved in deionized water to form a clear and transparent solution. This solution was named solution two. Solution two was added to solution one to form a clear and transparent solution, and the mixed solution was stirred at room temperature for 1 hour to fully mix the two solutions. The final mixture composition molar ratio is Al(NO 3 ) 3 9H 2 O:NaAC:H 2 O=1:2:139. The mixture was transferred to a stainless steel crystallization autoclave lined with polytetrafluoroethylene, and the crystallization autoclave was placed in an oven at 200 °C for 24 hours for hydrothermal treatment. After the hydrothermal treatment was completed, the solid product in the crystallization tank was centrifuged and washed three times with deionized water, and then the solid was dried...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com