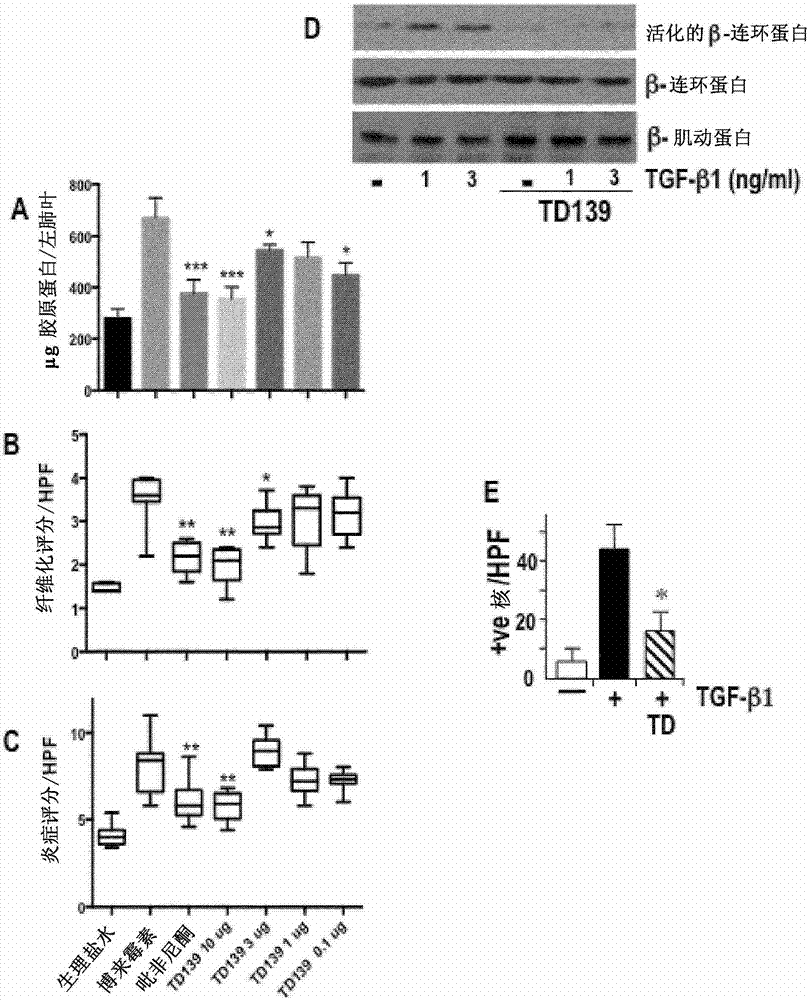
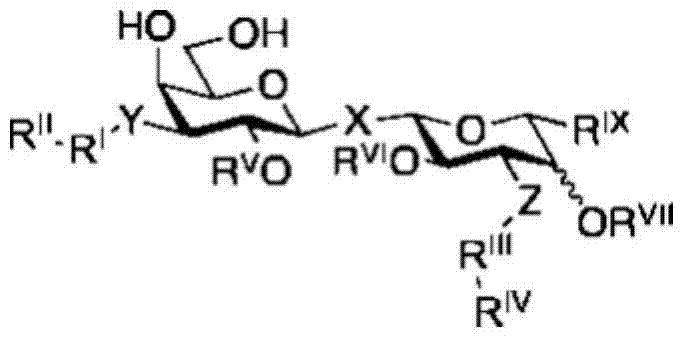
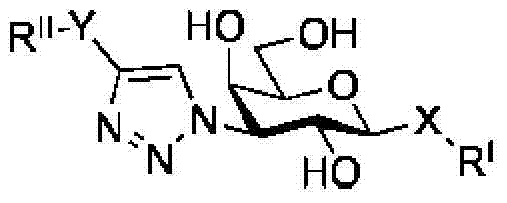
Galactoside inhibitor of galectin-3 and its use for treating pulmonary fibrosis
A technology of pulmonary fibrosis and liposome preparation, applied in the field of nebulizer and lung administration, which can solve the problems of unclear pulmonary fibrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0145] Compounds of formula (I) can be prepared as described in the experimental part below.
[0146] Therefore, there is provided a method for preparing a compound of formula I, comprising the steps of: making bis-(3-deoxy-3-azido-β-D-galactopyranosyl)sulfane with 3-fluorophenylacetylene and Reaction of an amine such as triethylamine, optionally in the presence of a catalyst such as Cu(I), in a solvent such as N,N-dimethylformamide (DMF) affords compounds of formula I. In a particular embodiment there is provided a process for the preparation of a compound of Formula I comprising the steps as described in Scheme 1 of the Experimental Section. Furthermore, the present invention relates to compounds of formula (I) obtainable by combining bis-(3-deoxy-3-azido-β-D-galactopyranosyl)sulfane with 3-fluorobenzene Reaction of an acetylene and an amine such as triethylamine, optionally in the presence of a catalyst such as Cu(I), in a solvent such as N,N-dimethylformamide (DMF) afford...
Embodiment 1
[0215] Example 1 Measuring Galectin-3 Levels in Human Lung Biopsies:
[0216] Biopsies were sampled from patients with Usual Interstitial Pneumonia (UIP, the most common cause of IPF). Biopsies were fixed in neutral buffered formalin for 12-24 h before embedding in solid paraffin for sectioning. Sections of 5um were cut and transferred to glass slides. Sections were deparaffinized in xylene for 10 min and rehydrated by placing slides in graded ethanol (100%-95%-80%-70%-50%-water) for 2 min each. Antigen retrieval was performed by microwave sectioning in 0.01 M citric acid pH 6.0 for 15 min. After cooling in running tap water, peroxidase was blocked by incubation in 1% hydrogen peroxide solution for 15 min. Slides were rinsed in phosphate-buffered saline (PBS), and non-specific binding was blocked using serum-free protein blocking and an avidin / biotin blocking kit (Vector Laboratories, USA). Sections were incubated overnight at 4°C with mouse monoclonal anti-human galectin-...
Embodiment 2
[0220] Example 2 Method for Measuring Galectin-3 Levels in Human Serum or Human Bronchoalveolar Lavage Fluid:
[0221] 1. Dilute the capture antibody to a working concentration in PBS without carrier protein. Immediately coat a 96-well microplate with 100 μL / well of diluted capture antibody. The plate was sealed and incubated overnight at room temperature.
[0222] 2. Aspirate wells and wash with wash buffer, repeat this process twice for a total of three washes. Wash by filling each well with wash buffer (400 μL) using a spray bottle, manifold dispenser, or automatic detergent. Complete removal of liquid at each step is essential for good performance. After the last wash, remove any residual wash buffer by aspirating or by inverting the plate and blotting with a clean paper towel.
[0223] 3. Block the plate by adding 300 [mu]L of reagent dilution to each well. Incubate at room temperature for at least 1 hour.
[0224] 4. Repeat the aspiration / washing as in step 2. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com