A disperse dye compound, and a preparing method and uses thereof
A technology of disperse dyes and compounds, applied in acylation preparation, dyeing method, organic dyes, etc., can solve the problems of low tinting strength, insufficient affinity, easy entry and exit, etc., to achieve improved affinity, increased van der Waals force, and increased contact face effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
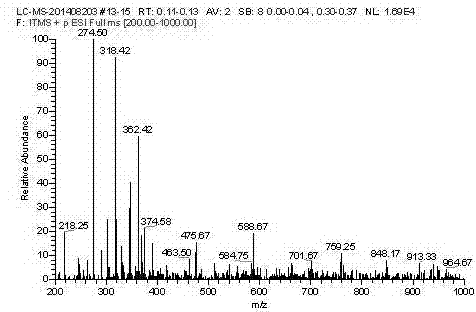
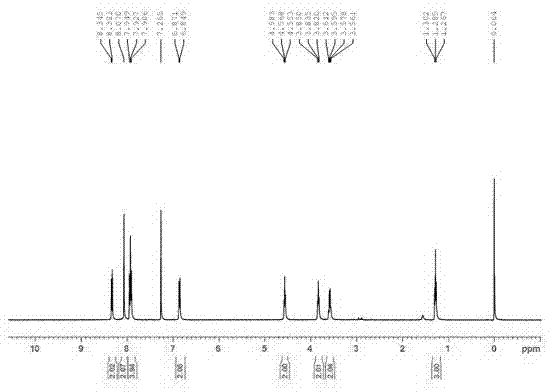
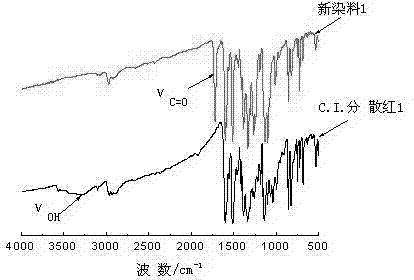
[0026] Add 2.50g of purified and vacuum-dried C.I. Disperse Red 1 dye to a 500ml three-necked flask, add an appropriate amount of dehydrated 300ml of dichloromethane as a solvent, stir to completely dissolve the disperse dye, add 1.60g and undergo dehydration treatment Accurately weigh 1.00g of diformyl chloride compound, dissolve and dilute it with an appropriate amount of reaction solvent, slowly drop it into a stirred reactor in an ice bath, and react at room temperature for 2-4h. Thin-layer sampling was used to track the reaction process. After the reaction was completed, 50ml of dilute acid solution was added, extracted and separated, the dye solution was rotary evaporated to remove the dichloromethane solvent, and then washed and filtered with an appropriate amount of sodium carbonate solution, and washed with water to obtain 2.89g of the crude product. The rate is 82.59%.
[0027] The crude product is recrystallized from dimethylformamide to obtain the pure product of t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com