Pharmaceutical composition of hydroxysafflor yellow A and salvianolic acid A, and kit
A technology of hydroxy safflower and composition, applied in the directions of drug combination, cardiovascular system diseases, etc., can solve the problems of impurity exclusion, allergic reaction, complex preparation components, etc., and achieves the effect of eliminating impurities, increasing the content, and eliminating allergic reactions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0031] 1. Test drug
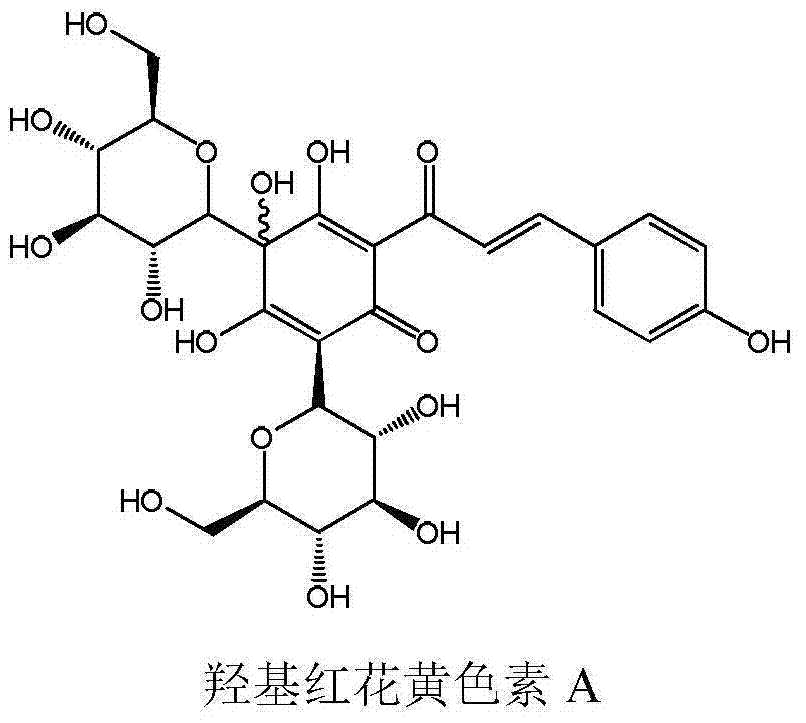
[0032] Hydroxysafflower yellow A: This product is yellow powder, provided by Zhejiang Yongning Pharmaceutical Co., Ltd.
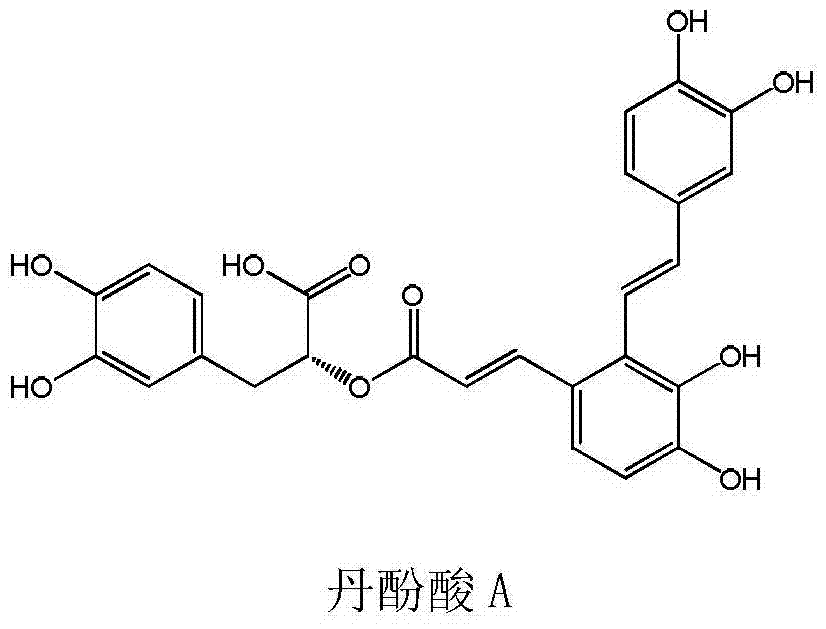
[0033] Salvianolic acid A: This product is light yellow powder, provided by Zhejiang Yongning Pharmaceutical Co., Ltd.
[0034] 2. Dose setting and grouping
[0035] 2.1. Single drug dose setting:
[0036] There are four doses of hydroxysafflower yellow A: 5mg / kg, 10mg / kg, 20mg / kg and 40mg / kg.
[0037] There are four doses of salvianolic acid A: 1.25mg / kg, 2.5mg / kg, 5mg / kg and 10mg / kg.
[0038] A normal saline control group was also set up.
[0039] 2.2. Compound ratio dose setting
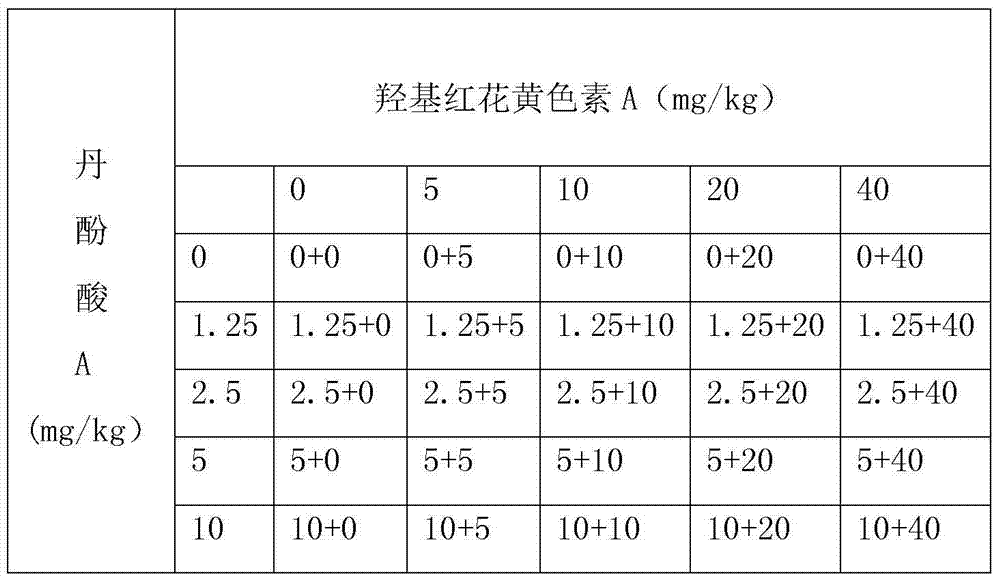
[0040] Hydroxysafflower yellow A and salvianolic acid A are arranged according to the Latin square, and there are 16 groups in total. There are 4 doses of each single drug combined with the solvent control group, a total of 25 groups (see Table 1), n=10 in each group.
[0041] Table 1 The ratio between different doses of hydroxysafflower yellow A and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com